Non-small cell lung cancer (NSCLC) represents a molecularly diverse group of malignancies where accurate biomarker identification directly determines treatment success. This resource details the complete molecular diagnostic pathway, from tissue acquisition and pathological subtyping through comprehensive genotyping and liquid biopsy applications, providing a systematic framework for implementing precision medicine in NSCLC management.
Overview of Non-Small Cell Lung Cancer (NSCLC)
Non-small cell lung cancer (NSCLC) represents the most prevalent form of lung cancer, accounting for approximately 85% of all cases and encompassing major subtypes such as adenocarcinoma, squamous cell carcinoma, and large cell carcinoma. Unlike its small cell counterpart, NSCLC typically demonstrates slower growth and spread but presents significant diagnostic complexity due to its heterogeneity. Modern diagnosis has evolved beyond simple histologic classification to require comprehensive molecular profiling for actionable biomarkers, including EGFR, ALK, and ROS1 alterations, that now define treatment pathways and enable personalized therapeutic strategies for advanced disease.
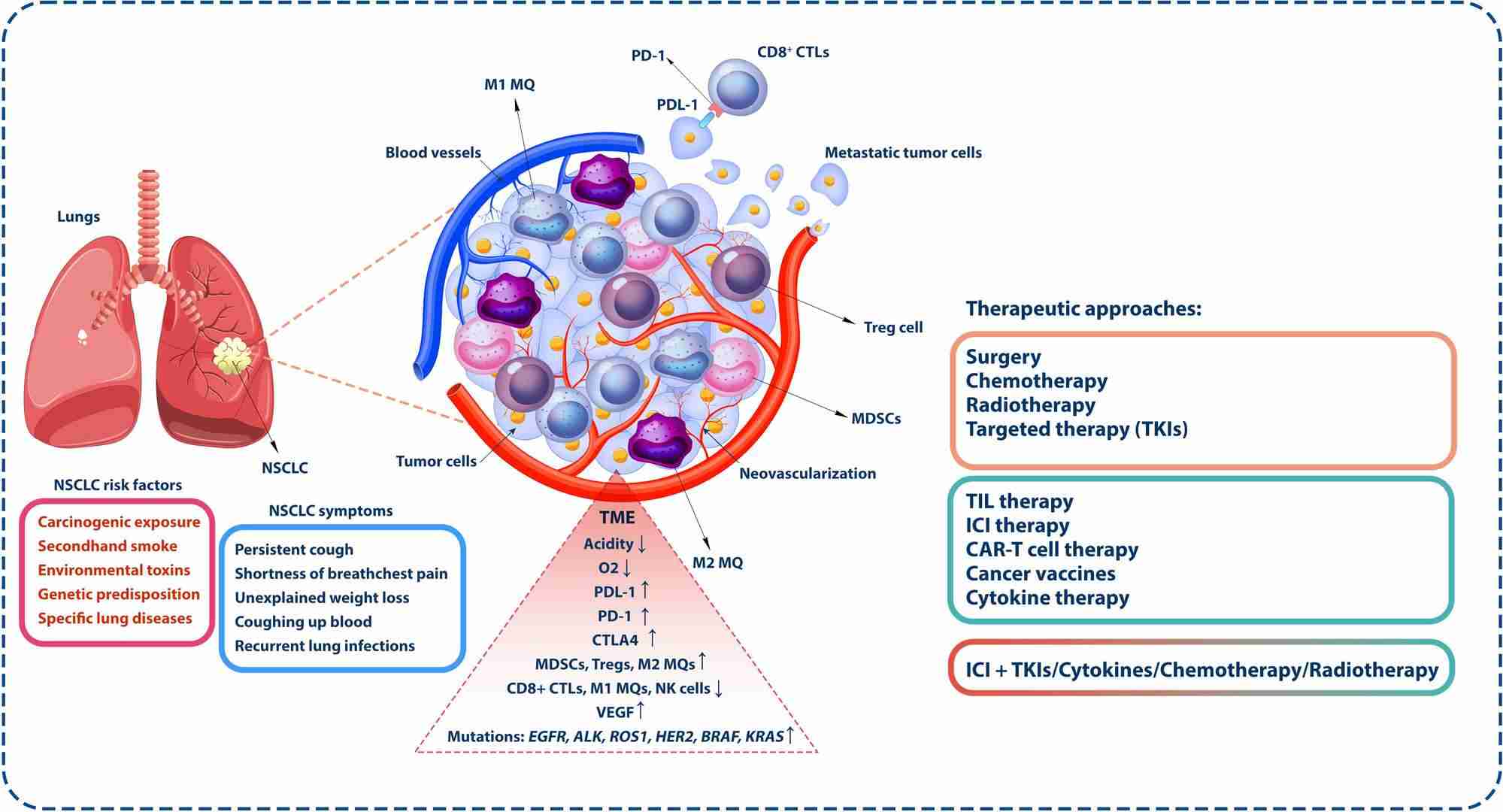 Fig.1 Tumor microenvironment in non-small cell lung cancer (NSCLC). (Wu, Yuanlin, et al., 2024)
Fig.1 Tumor microenvironment in non-small cell lung cancer (NSCLC). (Wu, Yuanlin, et al., 2024)
Nodule Detection and Characterization
The diagnostic pathway for non-small cell lung cancer (NSCLC) most commonly begins with the identification of a pulmonary nodule—a small, round or oval-shaped shadow in the lung tissue often discovered incidentally on medical imaging. This initial phase is critical, as its primary goal is to non-invasively distinguish between benign findings and potential malignancies, thereby determining which patients require further, more invasive investigation. The process relies heavily on advanced imaging technologies and standardized risk assessment models to ensure a systematic and evidence-based approach.
Computed Tomography (CT)
High-resolution computed tomography (CT) scanning serves as the cornerstone for nodule detection and initial characterization. It provides detailed, cross-sectional images of the lungs, allowing radiologists to assess critical morphological features of a nodule, including its size, density (solid, part-solid, or ground-glass), margins (spiculated or smooth), and growth rate over time. These characteristics are the first and most important clues in estimating the probability of malignancy.
Risk Stratification with Prediction Models
To move from subjective assessment to objective risk quantification, clinicians utilize validated risk prediction models. The Brock University model is one prominent example that integrates clinical factors (such as patient age, sex, and family history) with specific CT features (like nodule size, location, and spiculation) to calculate a percentage risk of malignancy. This calculated risk then directly informs consensus-based management guidelines, deciding whether a patient enters a program of active CT surveillance or proceeds directly to more advanced imaging or biopsy.
The Diagnostic Cornerstone: Tissue Acquisition and Pathological Confirmation
When imaging studies identify a suspicious pulmonary nodule, obtaining tissue samples for microscopic analysis becomes the definitive step in the diagnostic pathway. This phase transforms radiological suspicion into a concrete diagnosis, providing not only confirmation of malignancy but also critical information about the tumor's type and characteristics that will guide all subsequent treatment decisions. The process consists of two equally critical components: the biopsy procedure to secure the sample, and the pathological examination to interpret it.
Biopsy Techniques
The choice of biopsy technique is tailored to the nodule's location and characteristics. For central lesions and critical staging information, endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) allows for simultaneous sampling of lung masses and mediastinal lymph nodes. For peripheral nodules, computed tomography-guided transthoracic needle biopsy (CT-TTNB) provides high diagnostic yield, while advanced electromagnetic navigation bronchoscopy enables precise access to difficult-to-reach areas, ensuring adequate tissue acquisition for both diagnosis and molecular testing.
Pathological Diagnosis
Pathological analysis begins with precise classification of the tumor type, primarily distinguishing between adenocarcinoma and squamous cell carcinoma through histological examination. This initial characterization is supplemented and refined by immunohistochemistry (IHC), which uses specific antibodies to identify cellular markers that confirm the diagnosis and provide the foundation for subsequent molecular testing, ultimately delivering a comprehensive pathological profile essential for treatment planning.
Genotyping of Non-Small Cell Lung Cancer (NSCLC)
Genotyping refers to the process of identifying specific genetic alterations in a tumor's DNA that drive cancer growth. In NSCLC, this molecular profiling has become a standard and essential component of the diagnostic workup, particularly for advanced-stage disease. It moves beyond traditional histologic classification to define subsets of cancer based on their unique genetic fingerprints, enabling oncologists to select targeted therapies that specifically inhibit the function of these altered genes, thereby delivering more effective and personalized treatment. The genotyping process focuses on identifying established and emerging biomarkers with approved targeted therapies. These include:
- EGFR Mutations: The most common actionable alterations, particularly sensitizing mutations in exons 19 and 21, which predict response to EGFR tyrosine kinase inhibitors (e.g., osimertinib).
- ALK Fusions: Oncogenic rearrangements that make tumors highly sensitive to ALK inhibitors (e.g., alectinib, lorlatinib).
- ROS1 Fusions: Similar to ALK, these rearrangements are effectively targeted with specific inhibitors.
- BRAF V600E Mutations: A specific point mutation targeted by combination BRAF and MEK inhibitor therapy.
- Other Emerging Biomarkers: This includes alterations in KRAS G12C, MET, RET, and NTRK, for which effective targeted drugs are now available.
IVD Products for Non-Small Cell Lung Cancer (NSCLC)
Alta DiagnoTech specializes in molecular diagnostics for NSCLC, providing a complete portfolio of IVD solutions for comprehensive biomarker testing. Our products enable detailed molecular characterization, from initial tissue processing to multi-gene analysis, delivering the genomic insights needed to guide targeted therapy selection in advanced lung cancer. If you have related needs, please feel free to contact us for more information or product support.
| Product Name |
Technology |
Application |
| NSCLC Comprehensive Genotyping Panel |
Next-Generation Sequencing (NGS) |
Simultaneous detection of key biomarkers (EGFR, ALK, ROS1, BRAF, KRAS, MET, RET) from tissue samples |
| Liquid Biopsy NSCLC Monitoring Assay |
Cell-free DNA Analysis / NGS |
Detects and monitors EGFR mutations and other biomarkers from blood plasma |
| Rapid EGFR Mutation Detection Kit |
Real-time PCR |
Fast, sensitive detection of common EGFR sensitizing mutations (exon 19 del, L858R) |
| ALK/ROS1 Fusion FISH Detection Kit |
Fluorescence In Situ Hybridization |
Gold-standard detection of ALK and ROS1 gene rearrangements |
| RNA Fusion Detection Panel |
RNA Sequencing |
Direct detection of gene fusions (ALK, ROS1, RET, NTRK) at transcript level |
| TMB and MSI Analysis Panel |
NGS with Bioinformatics |
Comprehensive genomic profiling for immunotherapy biomarkers |
| NSCLC Tissue Nucleic Acid Preservation Kit |
Specialized Chemical Formulation |
Maintains DNA/RNA integrity in biopsy samples for optimal molecular testing |
Reference
- Wu, Yuanlin, et al. "Advancing non-small cell lung cancer treatment: the power of combination immunotherapies." Frontiers in Immunology 15 (2024): 1349502.
This article is for research use only. Do not use in any diagnostic or therapeutic application.



 Fig.1 Tumor microenvironment in non-small cell lung cancer (NSCLC). (Wu, Yuanlin, et al., 2024)
Fig.1 Tumor microenvironment in non-small cell lung cancer (NSCLC). (Wu, Yuanlin, et al., 2024)