Acinetobacter baumannii, a notorious multidrug-resistant pathogen, poses critical challenges in healthcare settings worldwide. This resource explores the evolving landscape of Acinetobacter diagnostics, from conventional methods struggling with prolonged turnaround times to cutting-edge technologies delivering same-day results. We examine how modern IVD solutions are transforming detection paradigms through molecular assays, advanced spectrometry, and genomic sequencing, while highlighting emerging innovations that promise to redefine diagnostic standards in the coming years.
Introduction to Acinetobacter Infection
Acinetobacter, particularly A. baumannii, is a formidable Gram-negative pathogen notorious for causing severe hospital-acquired infections (HAIs), including pneumonia, bloodstream infections, and wound infections. Recognized by the WHO as a “Critical Priority“ antibiotic-resistant pathogen, it thrives in healthcare settings, exhibiting rapid resistance development—especially to carbapenems.
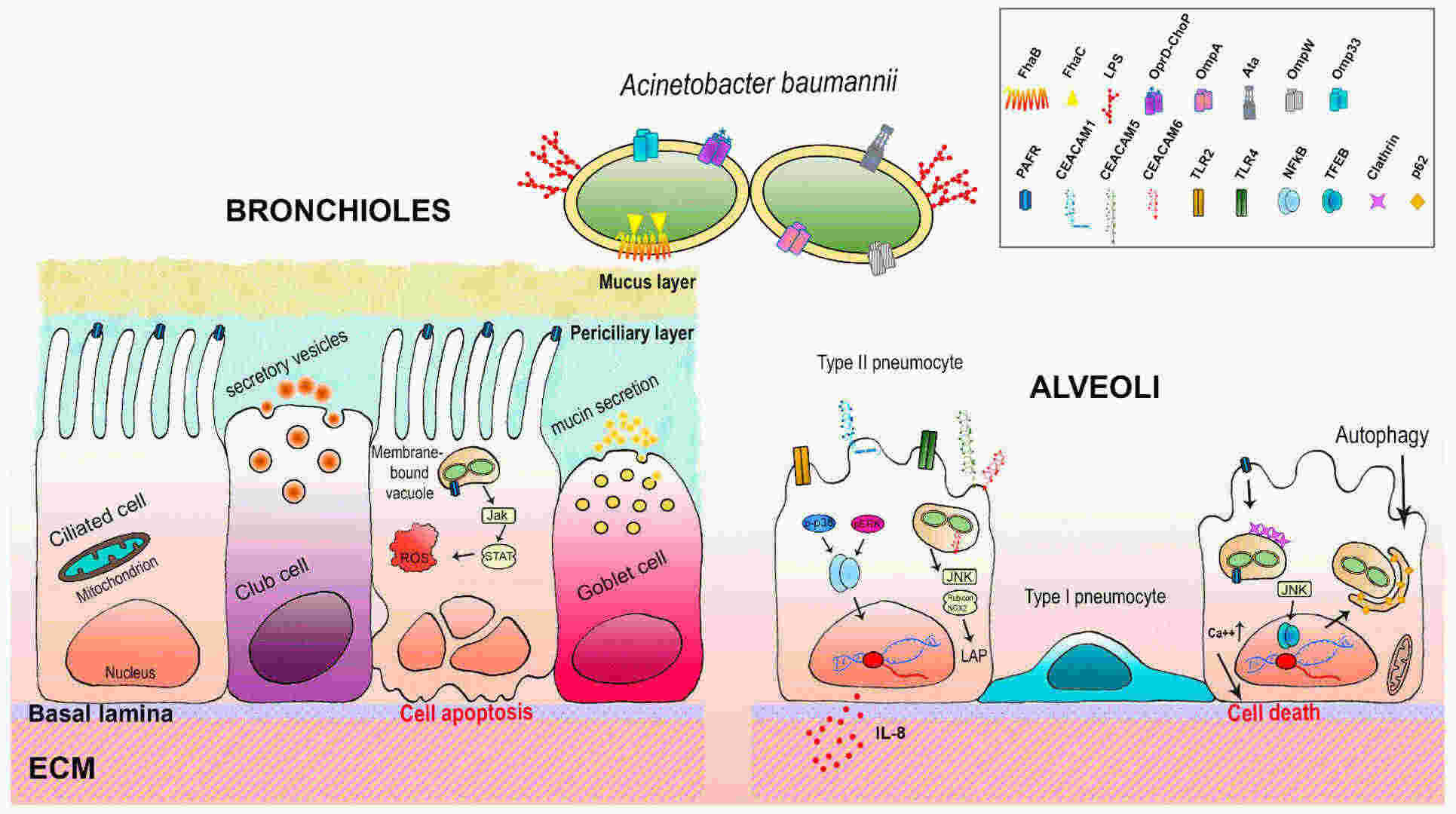 Fig.1 Schematic representation of the interrelations between A. baumannii and the respiratory epithelium. (Sarshar, Meysam, et al., 2021)
Fig.1 Schematic representation of the interrelations between A. baumannii and the respiratory epithelium. (Sarshar, Meysam, et al., 2021)
Diagnostic Challenges of Acinetobacter Infection
Rapid and precise diagnosis is essential to guide effective antimicrobial therapy, prevent transmission, and improve patient outcomes—especially in ICU and immunocompromised populations. Traditional diagnostic methods for Acinetobacter face critical limitations of prolonged turnaround times (3-5 days), variable sensitivity, and inability to rapidly detect emerging resistance mechanisms, often delaying life-saving therapeutic decisions.
| Traditional Methods |
Description |
Advantages |
Limitations |
| Cultures |
Growth-dependent isolation on selective media (e.g., blood agar). |
Gold standard for detection; enables downstream AST |
Slow (48–72 hours); low sensitivity if antibiotics were used, requires viable bacteria |
| Phenotypic AST |
Tests antibiotic effectiveness (e.g., disk diffusion, MIC assays). |
Provides actionable resistance data |
Adds 24–48 hours post-culture; may miss emerging resistance mechanisms |
| Biochemical Identification |
Identifies species via metabolic reactions. |
Low cost, widely available |
Misidentifies closely related species (e.g., A. baumannii vs. A. pittii); slow (24–48 hours) |
| Manual Microscopy |
Gram staining to visualize Gram-negative coccobacilli. |
Rapid (<30 mins), low cost |
Cannot differentiate Acinetobacter from other Gram-negative bacteria, low specificity |
Modern IVD Technologies for Acinetobacter Diagnostics
The rise of multidrug-resistant Acinetobacter strains demands faster, more precise diagnostic solutions. Modern IVD technologies now enable same-day detection, species identification, and comprehensive resistance profiling, overcoming the limitations of conventional methods.
Rapid Molecular Diagnostics
Technologies such as multiplex PCR and real-time PCR can detect Acinetobacter DNA and drug resistance genes (blaOXA-23/58, blaNDM) directly from clinical samples within 1-4 hours. Fully automated workstations integrate the sample-to-result workflow, delivering high sensitivity (approximately 95%) and specificity. These tools are crucial for early intervention in cases of sepsis or pneumonia.
Mass Spectrometry (MALDI-TOF MS)
MALDI-TOF MS provides rapid species identification (≤1 hour) from positive cultures by analyzing bacterial protein fingerprints. It outperforms biochemical methods in accuracy but requires prior culture growth and lacks direct resistance detection. Emerging protocols (e.g., direct specimen testing) aim to bypass culture, potentially transforming its utility.
Next-generation Sequencing (NGS)
NGS delivers whole-genome insights, identifying species, resistance genes, and strain lineages in a single assay. It’s invaluable for outbreak tracking and detecting novel resistance mechanisms, but high costs and bioinformatics barriers limit routine use. Portable sequencers may soon enable near-POC genomic resistance profiling.
Future Directions of Acinetobacter Diagnostics
The future of Acinetobacter diagnostics lies in integration, automation, and accessibility, with emerging technologies poised to overcome current challenges. AI-powered platforms will enable real-time resistance prediction from genomic or phenotypic data, while nanopore sequencing could deliver portable, culture-free detection of MDR strains within hours. Advances in non-invasive biomarkers (e.g., breath or urine VOCs) and microfluidics may enable true point-of-care testing, particularly for ICU and low-resource settings. Meanwhile, multi-omics approaches (proteomics, metabolomics) promise to uncover novel diagnostic signatures for early infection and treatment response monitoring.
Specializing in pioneering infectious disease IVD solutions, Alta DiagnoTech offers a comprehensive portfolio of Acinetobacter diagnostics, including rapid PCR test kits, carbapenemase test kits, and integrated workstations, to support faster and more intelligent clinical decision-making. If you have related needs, please feel free to contact us for more information or product support.
Reference
- Sarshar, Meysam, et al. “Acinetobacter baumannii: an ancient commensal with weapons of a pathogen.“ Pathogens 10.4 (2021): 387.
This article is for research use only. Do not use in any diagnostic or therapeutic application.



 Fig.1 Schematic representation of the interrelations between A. baumannii and the respiratory epithelium. (Sarshar, Meysam, et al., 2021)
Fig.1 Schematic representation of the interrelations between A. baumannii and the respiratory epithelium. (Sarshar, Meysam, et al., 2021)