- Home
- Resource
- Disease Diagnosis
- Infectious Diseases
- Poliomyelitis Diagnostics Decoded: From Lab Algorithms to Cutting-Edge Detection
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
As the world advances toward global polio eradication, reliable laboratory diagnostics remain critical for identifying outbreaks, distinguishing wild-type from vaccine-derived strains, and guiding public health action. This comprehensive resource outlines the complete diagnostic workflow, from clinical suspicion to confirmatory testing, and explores gold-standard methods for optimizing accuracy.
Poliomyelitis, commonly called polio, is a highly infectious viral disease caused by the poliovirus (serotypes 1, 2, or 3), which primarily targets the nervous system and can lead to irreversible paralysis. While global vaccination efforts have nearly eradicated wild poliovirus, challenges persist with vaccine-derived strains (VDPVs) and outbreaks in underimmunized populations. Rapid and accurate laboratory diagnosis is critical for surveillance, outbreak containment, and maintaining polio-free status.
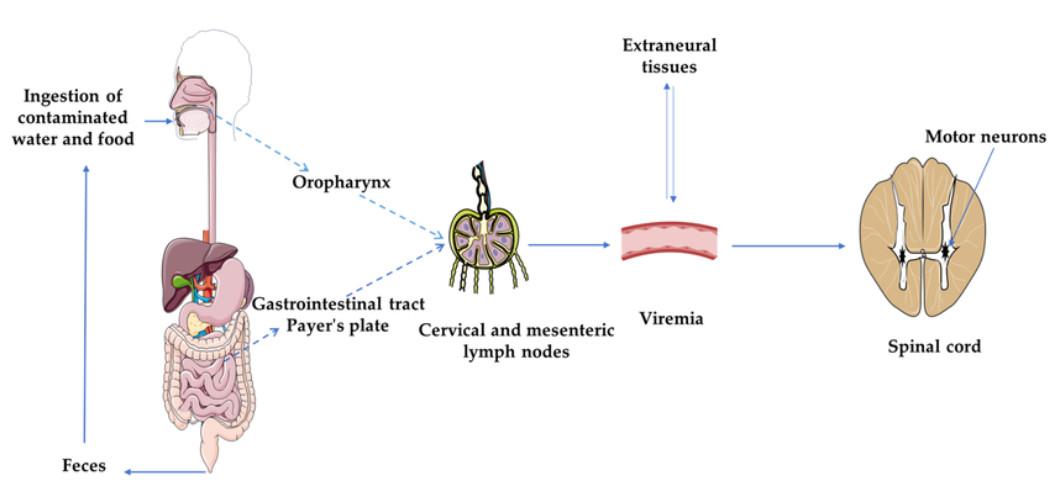 Fig.1 Pathogenesis of poliomyelitis. (Mbani C J, et al., 2023)
Fig.1 Pathogenesis of poliomyelitis. (Mbani C J, et al., 2023)
An efficient and accurate diagnostic workflow is essential for detecting poliovirus, confirming cases, and supporting global eradication efforts. The process integrates clinical assessment, sample collection, and laboratory testing to ensure timely and reliable results. Here's a step-by-step breakdown:
Clinical suspicion and case identification form the foundation of polio surveillance and outbreak control, serving as the primary trigger for laboratory testing and public health action.

Screening
The initial screening for poliovirus relies on real-time RT-PCR, which detects viral RNA in stool, CSF, or environmental samples within hours, which is critical for rapid response to epidemics. This method targets conserved regions like the VP1 capsid protein or 5’ UTR, offering high sensitivity to rule out polio quickly. In resource-limited settings, antigen detection assays (e.g., ELISA) may serve as preliminary tools, though they are less sensitive than molecular methods. Screening prioritizes speed to trigger public health actions while minimizing unnecessary culture-based testing.
Confirmation & Strain Differentiation
Positive screening results require confirmation and strain discrimination to identify wild-type, vaccine (Sabin), or vaccine-derived poliovirus (VDPV). The gold standard is virus isolation in cell cultures (e.g., L20B or RD cells), though this takes 7–10 days. Faster alternatives include intratypic differentiation (ITD) RT-PCR, which distinguishes strains by genetic markers. For outbreaks, whole-genome sequencing is added to track transmission chains and identify mutations. These steps are vital for guiding vaccination strategies.
Serology
Serological tests (e.g., neutralization assays or ELISA) detect poliovirus-specific IgM (acute infection) or IgG (past exposure/vaccination). While less useful for early diagnosis (antibodies take days to weeks to develop), they aid in:

Timely and accurate reporting of poliovirus detection is critical for global surveillance and outbreak containment. Laboratories must immediately notify national health authorities and the WHO Global Polio Laboratory Network (GPLN) upon positive results, especially for vaccine-derived (VDPV) or wild-type strains. Public health responses include emergency vaccination campaigns (e.g., ring vaccination), enhanced environmental monitoring, and contact tracing. Genetic sequencing data, when available, helps track transmission chains and guide targeted interventions. This step ensures alignment with the Global Polio Eradication Initiative (GPEI), turning lab findings into actionable strategies to prevent resurgence.
The future of poliovirus diagnostics is shifting toward ultra-sensitive, decentralized, and genomic-enabled technologies to support the final phase of global eradication. Key innovations include:
Alta DiagnoTech delivers end-to-end poliomyelitis diagnostics solutions, offering high-precision test kits, cutting-edge detection instruments, and optimized consumables to support global eradication efforts. If you have related needs, please feel free to contact us for more information or product support.
Reference
This article is for research use only. Do not use in any diagnostic or therapeutic application.
|
There is no product in your cart. |