- Home
- Resource
- Explore & Learn
- Pioneering the Path for Innovative Tuberculosis Diagnosis
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Tuberculosis (TB) remains one of the deadliest infectious diseases worldwide, despite being preventable and treatable. In 2021 alone, TB claimed approximately 1.5 million lives. Although TB is a well-known disease, its detection has long been hindered by ineffective diagnostic tools, particularly in low-resource settings. The COVID-19 pandemic, however, has spurred unprecedented advances in diagnostic technology, and these innovations could potentially reshape TB diagnosis. Lessons from the COVID-19 era offer valuable insights for tackling the ongoing diagnostic challenges in TB. This article delves into how COVID-19-driven innovations in diagnostics can be leveraged to revolutionize TB detection, enhancing both speed and accuracy.
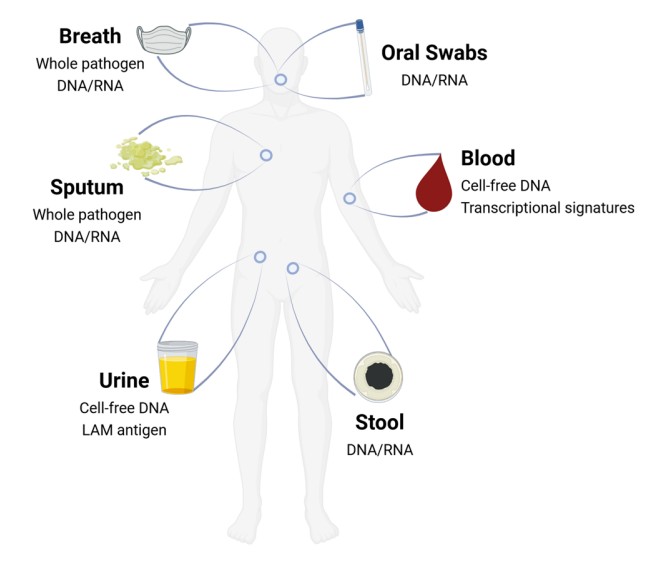 Fig.1 Clinical samples and targets proposed for TB testing. (Yerlikaya S., et al., 2024)
Fig.1 Clinical samples and targets proposed for TB testing. (Yerlikaya S., et al., 2024)

Current Diagnostics: Outdated and Inadequate
Sputum smear microscopy, the most widely used TB diagnostic method, has been a cornerstone of TB detection for decades. However, its limitations are evident, especially in populations like children or HIV-positive individuals, where sputum production is either difficult or insufficient. Additionally, smear microscopy has low sensitivity, often failing to detect TB in individuals with low bacterial loads. Despite improvements in molecular diagnostics like PCR, these methods are not universally accessible, especially in low-resource settings where the majority of TB cases are found.
TB diagnosis remains slow and labor-intensive, with many cases going undetected due to poor access to quality testing. A significant percentage of TB cases go undiagnosed every year, further exacerbating the global health crisis. With an estimated one-third of all TB cases going undiagnosed annually, new, more efficient methods are urgently needed.
The Need for Innovation in TB Diagnostics
The global TB diagnostic landscape has remained relatively stagnant, with few breakthroughs over the past few decades. Despite the availability of rapid molecular tests like the GeneXpert system, their widespread implementation is limited by high costs, infrastructure needs, and technical expertise required. There is a growing demand for diagnostic tools that are not only more accurate but also faster, cheaper, and easier to use, particularly in resource-poor settings.
COVID-19 diagnostics, on the other hand, experienced an extraordinary leap forward. The urgency of detecting and isolating COVID-19 cases led to the rapid development and deployment of a range of diagnostic tools, from molecular PCR tests to rapid antigen tests and even at-home testing kits. This wave of innovation provides a roadmap for overcoming the diagnostic barriers that have long plagued TB.
The rapid scale-up of COVID-19 diagnostic solutions was driven by significant investments and the urgency of managing a global pandemic. Governments and private companies around the world injected billions of dollars into diagnostic research and development. Within months, a variety of testing platforms, from PCR tests to lateral flow immunoassays, were developed and deployed worldwide. This speed was unprecedented in the history of diagnostic testing.
In the case of TB, the challenge has always been the slow pace of innovation. For example, the development of PCR-based diagnostics for TB was a gradual process, taking decades to refine and bring to market. In contrast, the COVID-19 pandemic showed that innovation could be accelerated, provided there was sufficient funding, global collaboration, and political will. The lessons learned from COVID-19 diagnostics must now be applied to TB to fast-track the development of novel, affordable, and accessible diagnostic technologies.

COVID-19 ushered in a new era of diagnostic platforms, such as portable PCR machines, antigen tests, and self-testing kits. These tests, often developed with minimal sample handling and rapid result turnaround times, dramatically changed how diagnostic testing is conducted. In TB, similar platforms could be developed using innovations from COVID-19 technology. For instance, point-of-care (POC) PCR systems like the TruenatTM and GeneXpert Omni are examples of portable diagnostic tools that could be adapted for TB testing in remote areas, where conventional lab-based testing is not feasible.
Additionally, the COVID-19 era saw the emergence of isothermal amplification techniques, such as Loop-mediated Isothermal Amplification (LAMP), which are less resource-intensive and offer faster results than traditional PCR methods. This type of innovation could be crucial in developing TB tests that can be performed at the point of care without requiring a complex laboratory setup.
The COVID-19 pandemic also accelerated the integration of artificial intelligence (AI) and machine learning (ML) into diagnostic tools. AI-based algorithms have been developed to analyze imaging data, such as chest X-rays, to detect COVID-19, often with greater accuracy than human radiologists. In TB, similar approaches could be used to analyze chest X-ray images, a critical diagnostic tool, to identify abnormalities linked to TB infection.
Moreover, digital health technologies that enable real-time data sharing and remote monitoring could significantly improve TB case management. These systems would allow healthcare workers to track patient data, monitor treatment progress, and make timely decisions. The COVID-19 era has shown that digital solutions can be integrated into healthcare systems to enhance both diagnostics and patient management. In TB, this could mean enhanced surveillance systems, AI-driven decision support, and mobile health apps for treatment adherence.
One of the major challenges in TB diagnostics is the collection of samples, particularly sputum, which is difficult for many patients to provide. The development of non-invasive diagnostic tests, as seen in the COVID-19 era, offers a solution to this problem. Nasal swabs, saliva samples, and even breath-based tests for COVID-19 have proven to be highly effective, reducing the burden on patients and healthcare systems. Similarly, oral swabs for TB testing have shown promising results. Recent studies have demonstrated that oral swabs, when processed with the Xpert MTB/RIF Ultra system, can yield TB results comparable to sputum-based tests, though further refinement is needed to optimize sensitivity.
Breath tests, which detect volatile organic compounds (VOCs) linked to TB infection, also hold promise. Although the technology is still in its infancy, COVID-19 has accelerated the development of breath-based diagnostic systems, and similar innovations could be applied to TB. The use of wearable devices or face masks equipped with sensors that detect aerosolized pathogens is another potential breakthrough that could allow for real-time TB diagnostics without the need for complex laboratory equipment.
The CRISPR-Cas9 technology, which gained significant attention during the COVID-19 pandemic for its role in rapid molecular diagnostics, could be leveraged for TB testing. CRISPR-based diagnostic systems offer a level of precision and speed that traditional PCR assays cannot match. By targeting specific genetic markers of Mycobacterium tuberculosis, CRISPR diagnostics could provide faster, cheaper, and more accessible testing solutions for TB. These tests could be used at the point of care, bypassing the need for complex laboratory equipment and skilled technicians.
Furthermore, CRISPR-based diagnostics could be integrated with isothermal amplification methods like LAMP, making them even more suitable for low-resource settings. The combination of CRISPR and LAMP offers the potential for a highly sensitive, portable, and affordable diagnostic tool for TB, which could be deployed in community health centers, remote areas, and even in at-home settings.
While the COVID-19 pandemic spurred massive investments into diagnostics, TB has historically received far less attention from global funding bodies. The WHO has set a target of US$2 billion annually for TB research, but funding has remained significantly lower. In 2020, global investment in TB research was only about US$915 million, less than half of the target amount. To match the speed and scale of COVID-19 diagnostic innovations, TB diagnostics must be prioritized with similar levels of financial support.
Scaling up TB diagnostics in low- and middle-income countries (LMICs) will require extensive collaboration between governments, private sector companies, and global health organizations. The success of COVID-19 diagnostics in LMICs was largely due to partnerships between organizations like FIND, the Stop TB Partnership, and national health ministries. Similar collaborative frameworks will be necessary to bring new TB diagnostics to high-burden countries.
Moreover, global initiatives such as the GDF (Global Drug Facility) and USAID's SMART4TB program are already working to support TB diagnostic innovation and implementation. These platforms provide invaluable access to clinical samples, clinical evaluations, and funding for R&D. Ensuring that these resources are utilized effectively will be crucial to the rapid adoption of new diagnostic technologies.
The COVID-19 pandemic has proven that innovation in diagnostic technologies can be achieved at an unprecedented pace. With lessons learned from this crisis, it is now possible to reimagine TB diagnostics with the potential to address long-standing challenges. From non-invasive sample collection methods to the integration of AI and CRISPR-based technologies, the future of TB diagnostics is bright. However, to fully capitalize on these innovations, there must be sustained investment in research and development, particularly in low-resource settings where the need is greatest.
By combining the technological advancements seen in COVID-19 diagnostics with the global collaboration and funding necessary to tackle TB, we have the opportunity to revolutionize TB detection and ultimately reduce the global burden of this devastating disease.
If you have related needs, please feel free to contact us for more information or product support.
Reference
This article is for research use only. Do not use in any diagnostic or therapeutic application.

Cat.No. GP-DQL-00203
Rotavirus Antigen Group A and Adenovirus Antigen Rapid Test Kit (Colloidal Gold)

Cat.No. GP-DQL-00206
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Card Style

Cat.No. GP-DQL-00207
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. GP-DQL-00211
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. GP-DQL-00212
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. IP-00189
Influenza A Rapid Assay Kit

Cat.No. GH-DQL-00200
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00201
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00202
Luteinizing Hormone Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00208
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. GH-DQL-00209
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit(Colloidal Gold), Strip Style

Cat.No. GH-DQL-00210
Luteinizing Hormone Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. IH-HYW-0001
hCG Pregnancy Test Strip

Cat.No. IH-HYW-0002
hCG Pregnancy Test Cassette

Cat.No. IH-HYW-0003
hCG Pregnancy Test Midstream

Cat.No. GD-QCY-0001
Cocaine (COC) Rapid Test Kit

Cat.No. GD-QCY-0002
Marijuana (THC) Rapid Test Kit

Cat.No. GD-QCY-0003
Morphine (MOP300) Rapid Test Kit

Cat.No. GD-QCY-0004
Methamphetamine (MET) Rapid Test Kit

Cat.No. GD-QCY-0005
Methylenedioxymethamphetamine ecstasy (MDMA) Rapid Test Kit

Cat.No. GD-QCY-0006
Amphetamine (AMP) Rapid Test Kit

Cat.No. GD-QCY-0007
Barbiturates (BAR) Rapid Test Kit

Cat.No. GD-QCY-0008
Benzodiazepines (BZO) Rapid Test Kit

Cat.No. GD-QCY-0009
Methadone (MTD) Rapid Test Kit

Cat.No. GD-QCY-0011
Opiate (OPI) Rapid Test Kit

Cat.No. ID-HYW-0002
Multi-Drug Test L-Cup, (5-16 Para)

Cat.No. ID-HYW-0005
Multi-Drug Rapid Test (Dipcard & Cup) with Fentanyl

Cat.No. ID-HYW-0006
Multi-Drug Rapid Test (Dipcard & Cup) without Fentanyl

Cat.No. ID-HYW-0007
Multi-Drug 2~14 Drugs Rapid Test (Dipstick & Dipcard & Cup)

Cat.No. ID-HYW-0008
Fentanyl (FYL) Rapid Test (For Prescription Use)

Cat.No. ID-HYW-0009
Fentanyl Urine Test Cassette (CLIA Waived)

Cat.No. ID-HYW-0010
Fentanyl Urine Test Cassette (Home Use)
|
There is no product in your cart. |