- Home
- Resource
- Disease Diagnosis
- Infectious Diseases
- Navigating Zika Virus Diagnosis: A Time-Sensitive Guide to Molecular and Serologic Testing
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Zika virus infection is a mosquito-borne disease with significant public health implications, particularly due to its potential to cause severe birth defects. This resource provides a clear, step-by-step guide to its time-sensitive diagnosis, detailing the critical key biomarkers and their detection windows, explaining the core molecular and serologic testing technologies, and outlining the definitive stepwise diagnostic algorithm based on symptom onset to ensure accurate detection and appropriate patient management.
Zika virus infection is a mosquito-borne arboviral disease caused by the Zika virus (ZIKV), a member of the Flaviviridae family, which also includes dengue and yellow fever viruses. While infection is often asymptomatic or causes mild, self-limiting symptoms (fever, rash, joint pain), its primary public health significance lies in its teratogenic effects; maternal infection during pregnancy can lead to severe birth defects, most notably microcephaly and other congenital Zika syndrome disorders. Diagnosis is challenging due to cross-reactivity with related flaviviruses and is highly time-sensitive, requiring appropriate selection of molecular (viral RNA) or serologic (IgM antibody) tests based on the precise timing of symptom onset.
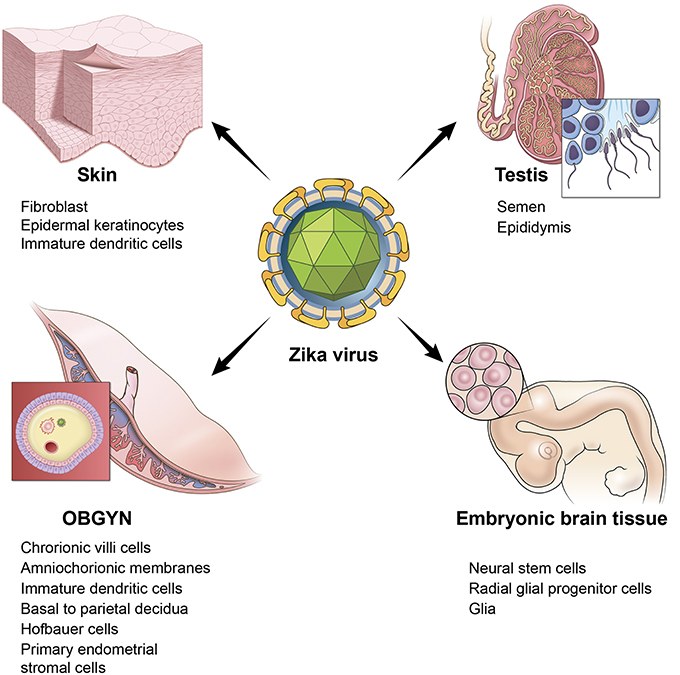 Fig.1 The Zika virus can infect various tissues in the human body. (Gorshkov, Kirill, et al., 2019)
Fig.1 The Zika virus can infect various tissues in the human body. (Gorshkov, Kirill, et al., 2019)
The accurate diagnosis of Zika virus infection hinges on detecting specific biomarkers whose presence in the body changes dramatically over time. Understanding when these markers appear and disappear is the most critical factor in selecting the correct diagnostic test and interpreting its result.
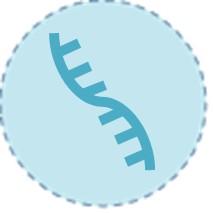
Viral RNA
Viral RNA is the genetic material of the Zika virus and is the direct biomarker of an active infection. It is detectable in the patient's blood for only a brief window, typically 1 to 2 weeks after symptom onset, and may persist slightly longer in urine. A positive RNA test confirms a current, acute Zika virus infection.
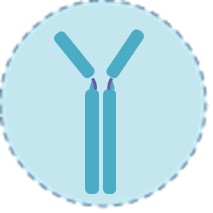
Zika Virus IgM Antibodies
Zika virus IgM antibodies are proteins produced by the immune system in response to the infection. They begin to rise towards the end of the first week and are the primary detectable biomarker from about 2 weeks to several months after infection. A positive IgM test indicates a recent or past infection, but due to significant cross-reactivity with antibodies from related viruses (like dengue), it often requires confirmatory testing.
Laboratory confirmation of Zika virus infection relies on a distinct set of technologies, each designed to detect a specific biomarker during its optimal time window. The choice between these core methods is not arbitrary but is fundamentally dictated by the stage of infection and the clinical question at hand.

Molecular testing for viral RNA, primarily using real-time reverse transcription polymerase chain reaction (rRT-PCR), is the method for directly detecting the virus's genetic material. It offers high specificity for diagnosing an acute, ongoing infection but is only reliable if the patient is tested within the narrow viremic window (typically <14 days after symptom onset).

Serologic testing for IgM antibodies, most commonly performed via enzyme-linked immunosorbent assay (ELISA), detects the immune system's response to the virus. This method extends the diagnostic window beyond the period of detectable viremia but faces the major challenge of significant cross-reactivity with antibodies from other flavivirus infections (e.g., dengue), which can lead to false-positive results and necessitate confirmatory neutralization tests.
The cornerstone of effective Zika virus diagnosis is a stepwise testing algorithm that is entirely driven by one critical variable: the time elapsed since the onset of symptoms. This time-based framework ensures the correct test is applied at the right moment to maximize the chances of an accurate result.

For Patients with Symptoms Onset < 14 Days
For symptomatic patients presenting within two weeks of symptom onset, the diagnostic pathway prioritizes the detection of active virus. The recommended first step is to collect both serum and urine specimens for Zika virus nucleic acid amplification testing (NAAT, e.g., RT-PCR). A positive result definitively confirms an acute Zika virus infection. If the NAAT is negative, testing of the same serum sample for Zika virus IgM antibodies should be performed to capture a potential early immune response.

For Patients with Symptoms Onset≥14 Days
For patients who present two weeks or more after symptoms began (or for asymptomatic individuals with exposure risk), the diagnostic window for detecting viral RNA has typically closed. Therefore, the primary and most appropriate test is a Zika virus IgM antibody assay on a serum sample. A positive IgM result provides presumptive evidence of a recent infection but, due to the possibility of cross-reactivity, often requires confirmation with a more specific test like the plaque reduction neutralization test (PRNT). A negative IgM result at this stage makes a recent Zika virus infection unlikely.
Beyond the standard testing algorithm, accurate diagnosis of Zika virus infection requires addressing several high-stakes complexities. These include the critical management of pregnant patients due to fetal risks, the resolution of ambiguous serology results caused by antibody cross-reactivity, and the essential practice of ruling out other clinically similar infections.
Alta DiagnoTech offers a comprehensive suite of in vitro diagnostic (IVD) solutions to address the complexity and time-sensitive challenges of diagnosing Zika virus infection. Our products support the entire diagnostic process, from rapid molecular detection of acute infection to specific serological confirmation, helping laboratories deliver accurate and actionable test results to support critical patient management and public health decisions. If you have related needs, please feel free to contact us for more information or product support.
| Product Name | Technology | Application |
| Zika Virus Real-Time RT-PCR Detection Kit | Real-Time Reverse Transcription Polymerase Chain Reaction (rRT-PCR) | Qualitative detection of Zika virus RNA in human serum, plasma, urine, or cerebrospinal fluid for the diagnosis of acute infection within approximately 14 days of symptom onset. |
| Zika Virus IgM Antibody ELISA Kit | Enzyme-Linked Immunosorbent Assay (ELISA) | Detection of Zika virus-specific IgM antibodies in human serum or plasma for the diagnosis of recent infection, typically used for specimens collected ≥14 days after symptom onset or from asymptomatic at-risk individuals. |
| Multiplex Arbovirus Molecular Panel (Zika, Dengue, Chikungunya) | Multiplex Real-Time RT-PCR | Simultaneous differential detection and identification of Zika, Dengue, and Chikungunya virus RNA from a single patient sample, crucial for differential diagnosis in regions where these arboviruses co-circulate. |
| Automated Flavivirus Serology Panel System | Chemiluminescent Immunoassay (CLIA) on an Automated Platform | High-throughput, automated screening for IgM and IgG antibodies against Zika and other relevant flaviviruses (e.g., Dengue), aiding in initial serological profiling and workflow efficiency. |
| Flavivirus Antibody Differentiation / PRNT Support Assay | Micro-Neutralization Test | Supplemental, high-specificity testing to help confirm positive Zika IgM results and differentiate Zika virus antibodies from cross-reacting antibodies caused by other flavivirus infections or vaccinations. |
Reference
This article is for research use only. Do not use in any diagnostic or therapeutic application.
|
There is no product in your cart. |