- Home
- Resource
- Explore & Learn
- Navigating the Maze of Microbial Pathogen Testing: A Comprehensive Guide to Selecting the Right Strains
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
In the intricate world of in vitro diagnostics (IVD), particularly within the realm of food safety and microbial pathogen detection, the selection of appropriate strains for method validation and verification stands as a cornerstone of accuracy and reliability. The ability to detect and quantify pathogens in diverse food matrices is not only a regulatory requirement but also a public health imperative. This guide delves into the nuanced process of selecting the right strains for evaluating rapid pathogen test methods, ensuring that laboratories can navigate the maze of microbial pathogen testing with confidence.
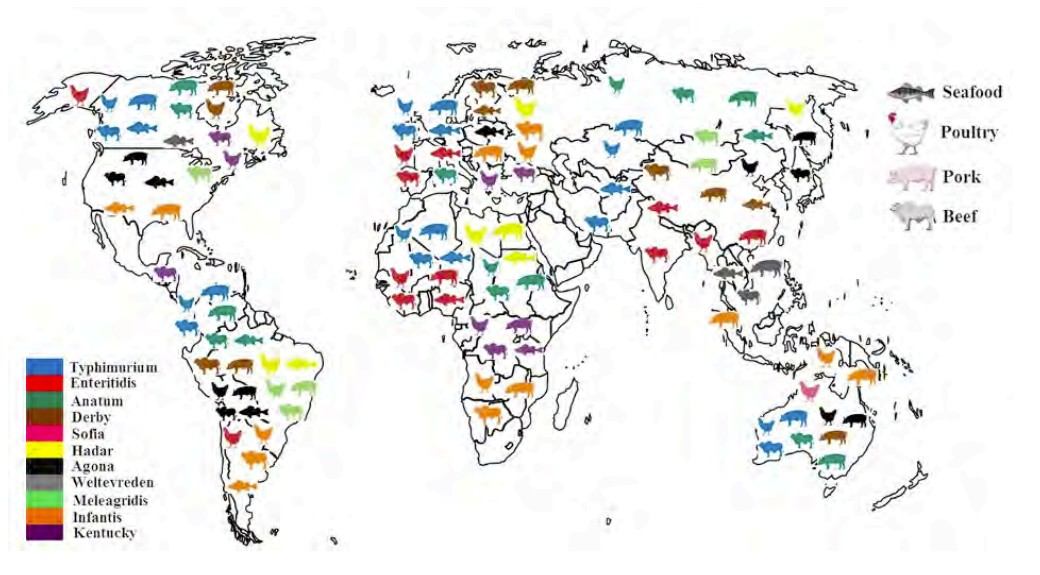 Fig.1 Worldwide distribution of Salmonella by Matrix. (Legan J. D., et al., 2022)
Fig.1 Worldwide distribution of Salmonella by Matrix. (Legan J. D., et al., 2022)

Process Environment: The Influence of Production Facilities
The processing environment is a critical factor that shapes the microbial ecology of food products. Certain pathogen subtypes are particularly well-adapted to persist in processing facilities, leading to recurrent contamination of products. For example, Listeria monocytogenes outbreaks in ready-to-eat (RTE) foods are often traced back to post-process contamination from the production environment. These persistent strains may exhibit resistance to cleaning and sanitization procedures, making them particularly challenging to eliminate.
When selecting strains for method validation, it is essential to consider those known to persist in similar processing environments. This approach ensures that the validation process accounts for the robustness of these strains, thereby enhancing the reliability of the detection method in real-world scenarios.

Geographical and Seasonal Considerations: A Global Perspective
The prevalence and distribution of pathogens are not uniform across geographical regions. For instance, the diversity of Salmonella serotypes in poultry products varies significantly between Asia, Latin America, Europe, and Africa. Additionally, seasonal fluctuations in pathogen incidence, driven by environmental factors and human activities, further complicate the landscape.
When selecting strains for method validation, it is crucial to consider the geographical origin and seasonal prevalence of the target pathogen. This approach ensures that the method is applicable across different regions and times of the year, providing a more comprehensive and reliable validation process.

Matrix Characteristics: Tailoring Strain Selection to Food Type
The physical and chemical properties of food matrices can significantly influence pathogen behavior. For example, the high fat content and low water activity (a_w) of chocolate can affect the survival and detectability of Salmonella. When selecting strains for method validation in chocolate, it is essential to choose those capable of withstanding these environmental stresses.
Similarly, for leafy greens, which are often associated with E. coli O157:H7 outbreaks, strains with a propensity for adhering to plant surfaces and resisting wash steps should be prioritized. This tailored approach ensures that the validation process accounts for the specific challenges posed by different food matrices, enhancing the accuracy and reliability of the detection method.

Clinical Considerations and Outbreak Data: Prioritizing Public Health Impact
The clinical severity of pathogen-induced illnesses varies significantly among serotypes. For example, Salmonella Enteritidis and Heidelberg are associated with higher case fatality rates compared to other serotypes. When selecting strains for method validation, prioritizing those linked to severe clinical outcomes ensures that the method can effectively detect the most virulent pathogens.
Additionally, incorporating strains implicated in previous outbreaks provides valuable insights into the method's performance under real-world conditions. This approach not only enhances the reliability of the detection method but also prioritizes public health impact by focusing on the most critical pathogens.
If you have related needs, please feel free to contact us for more information or product support.
Reference
This article is for research use only. Do not use in any diagnostic or therapeutic application.
|
There is no product in your cart. |