- Home
- Resource
- Explore & Learn
- Navigating Hurdles and Innovations in Monoclonal Antibody-Based Diagnostics
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
In the dynamic realm of modern medicine, in vitro diagnosis (IVD) plays a pivotal role, influencing over 70% of clinical decisions while consuming a mere 3% of medical resources. At the core of this crucial field are diagnostic monoclonal antibodies. These specialized proteins function as molecular detectives, identifying specific biomarkers to detect diseases, monitor treatment responses, and guide clinical decision-making.
The global IVD market reached approximately 70 billion USD in 2019, with China's market contributing around 86.4 billion RMB, constituting about 15% of the global total. The COVID-19 pandemic catapulted this sector into the spotlight, with China's antigen testing market alone projected to exceed 100 billion RMB annually. However, despite this growth, China's IVD industry grapples with a significant challenge: a heavy reliance on imported core raw materials, including 70% of critical biological materials such as antigens and antibodies. This dependence not only inflates costs but also poses potential risks to healthcare security.
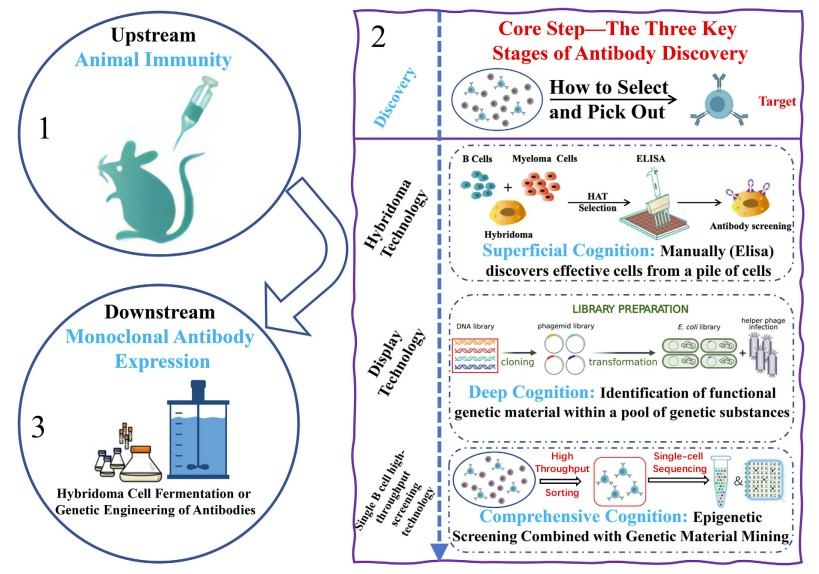 Fig.1 Three critical phases of antibody discovery: i) superficial cognition: manually (Elisa) discovers effective cells from a pile of cells; ii) deep cognition: identification of functional genetic material within a pool of genetic substances; iii) comprehensive cognition: epigenetic screening combined with genetic material mining. (Wang J., et al., 2024)
Fig.1 Three critical phases of antibody discovery: i) superficial cognition: manually (Elisa) discovers effective cells from a pile of cells; ii) deep cognition: identification of functional genetic material within a pool of genetic substances; iii) comprehensive cognition: epigenetic screening combined with genetic material mining. (Wang J., et al., 2024)
The journey of antibody discovery spans over a century, punctuated by groundbreaking innovations that have revolutionized healthcare.
The Early Foundations (1890-1970s)
In 1890, German scientist Emil Adolf von Behring and Japanese scientist Kitasato Shibasaburo made a momentous discovery: serum from rabbits infected with tetanus could shield mice from the disease. This marked the inception of antitoxin research and laid the groundwork for understanding humoral immunity.
Paul Ehrlich expanded on this in 1897 with his side-chain theory, positing that cell-surface receptors specifically bind to toxins, triggering antibody production. This concept of specificity remains central to immunology to this day.
The 1920s witnessed Michael Heidelberger and Oswald Theodore Avery confirm that antibodies are proteins, while the 1930s brought John Marrack's antigen-antibody binding theory, elucidating the biochemical characteristics of this interaction.
A major breakthrough occurred in 1959 when Gerald Maurice Edelman and Porter deciphered antibody structure, identifying heavy and light chains and the crucial antigen-binding sites. This work earned them the 1972 Nobel Prize in Physiology or Medicine.
The Monoclonal Revolution (1970s-1990s)
1975 was a turning-point when Georges Köhler and César Milstein invented hybridoma technology, fusing antibody-producing cells with cancer cells to create immortalized lines that produce identical monoclonal antibodies. This breakthrough revolutionized antibody production.
Susumu Tonegawa's 1976 discovery of antibody diversity through gene rearrangement (V, D, J genes) further advanced our understanding, explaining how the immune system generates a vast array of antibodies.
The 1980s introduced phage display technology, developed by Smith, allowing researchers to display peptides or antibodies on phage surfaces for rapid screening. This technique, advanced by Winter, led to the first fully human monoclonal antibody drug.
Modern Innovations (2000s-Present)
Recent years have seen the emergence of single B-cell screening combined with single-cell sequencing, enabling efficient isolation of specific antibody-producing cells and their genetic sequences. This approach allows for "digital preservation" of antibodies and stable production.
Today, we stand on the cusp of another revolution: artificial intelligence (AI)-driven antibody design, which holds the promise of bypassing traditional methods entirely by generating new antibody sequences using computational algorithms.
The IVD Ecosystem
The in vitro diagnostic industry operates within a complex chain:
Antibodies, as critical upstream components, significantly impact diagnostic accuracy and cost. However, China's IVD sector faces substantial challenges in this area.
Key Challenges in Diagnostic Antibody Development
-High R & D difficulty for key raw materials
-Stringent production requirements affecting accuracy, sensitivity, and specificity
-Limited product variety and batch-to-batch variations in domestic production
Animal Immunization: The Starting Point
The process commences with immunizing animals to generate an immune response. Key challenges here include:
Antibody Screening: Finding the Needle in the Haystack
Identifying B cells that produce effective antibodies is a formidable challenge. Three main approaches have been developed:
Hybridoma Technology: Fuses antibody-producing cells with cancer cells to create immortalized lines. While pioneering, this method has drawbacks:
Phage Display Systems: Displays antibodies on phage surfaces for screening. This method:
Single B-Cell Screening: Utilizes microfluidics and nanopore technologies to isolate individual B cells. Combined with single-cell sequencing:
Antibody Expression and Production
Once identified, antibodies must be produced at scale. This process presents its own set of challenges:
-Bacteria (E. coli) can only produce antibody fragments, not full antibodies
-Yeast, insect, and plant cells perform post-translational modifications differently than human cells
-Only mammalian cells produce antibodies with human-like modifications, making them the gold standard despite higher costs
To surmount these challenges, researchers are developing groundbreaking approaches:

Advanced Immunization Techniques
Drawing inspiration from mRNA vaccines, researchers are exploring mRNA-based immunization:

AI-Driven Antibody Discovery
Computational approaches are revolutionizing antibody development:
This "biotechnology and computer information technology" (BTIT) fusion significantly reduces validation costs and accelerates development.
Modular Antibody Design
Taking cues from therapeutic antibody advancements, diagnostic antibodies are moving towards modular, customized designs:
These designs can enhance sensitivity, reduce interference, and simplify diagnostic processes.
Protein Mass Spectrometry Sequencing
This technology offers a shortcut by directly sequencing antibodies from serum:
While powerful, this approach raises concerns about intellectual property and may discourage original development if misused.
The development of diagnostic antibodies stands at a crossroads. On one hand, technologies like AI-driven design and mRNA immunization hold the promise of revolutionizing the field. On the other hand, practical challenges of cost, validation, and intellectual property must be addressed.
The "Spiegelman's Monster" phenomenon serves as a cautionary tale: in competitive environments, the simplest, fastest-replicating entities often dominate, potentially crowding out more effective but complex solutions. This risk exists in antibody development, where easy reverse-engineering might discourage investment in innovative approaches.
To avoid this pitfall, the industry must:
The field of diagnostic monoclonal antibodies has come a long way since the early days of serum therapy. From hybridomas to AI design, each advancement has brought us closer to more precise, efficient, and accessible diagnostics.
As we peer into the future, the integration of biotechnology with computational approaches will be the linchpin. By enhancing immunization efficiency, improving screening methods, and reimagining antibody structures, researchers are laying the groundwork for a new generation of diagnostic tools.
The journey from serum antitoxins to AI-designed antibodies represents one of the most remarkable sagas in modern biotechnology. As we continue this journey, the potential to revolutionize healthcare through better diagnostics remains both our greatest challenge and our most promising opportunity.
If you have related needs, please feel free to contact us for more information or product support.
Reference
This article is for research use only. Do not use in any diagnostic or therapeutic application.

Cat.No. GP-DQL-00203
Rotavirus Antigen Group A and Adenovirus Antigen Rapid Test Kit (Colloidal Gold)

Cat.No. GP-DQL-00206
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Card Style

Cat.No. GP-DQL-00207
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. GP-DQL-00211
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. GP-DQL-00212
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. IP-00189
Influenza A Rapid Assay Kit

Cat.No. GH-DQL-00200
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00201
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00202
Luteinizing Hormone Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00208
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. GH-DQL-00209
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit(Colloidal Gold), Strip Style

Cat.No. GH-DQL-00210
Luteinizing Hormone Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. IH-HYW-0001
hCG Pregnancy Test Strip

Cat.No. IH-HYW-0002
hCG Pregnancy Test Cassette

Cat.No. IH-HYW-0003
hCG Pregnancy Test Midstream

Cat.No. GD-QCY-0001
Cocaine (COC) Rapid Test Kit

Cat.No. GD-QCY-0002
Marijuana (THC) Rapid Test Kit

Cat.No. GD-QCY-0003
Morphine (MOP300) Rapid Test Kit

Cat.No. GD-QCY-0004
Methamphetamine (MET) Rapid Test Kit

Cat.No. GD-QCY-0005
Methylenedioxymethamphetamine ecstasy (MDMA) Rapid Test Kit

Cat.No. GD-QCY-0006
Amphetamine (AMP) Rapid Test Kit

Cat.No. GD-QCY-0007
Barbiturates (BAR) Rapid Test Kit

Cat.No. GD-QCY-0008
Benzodiazepines (BZO) Rapid Test Kit

Cat.No. GD-QCY-0009
Methadone (MTD) Rapid Test Kit

Cat.No. GD-QCY-0011
Opiate (OPI) Rapid Test Kit

Cat.No. ID-HYW-0002
Multi-Drug Test L-Cup, (5-16 Para)

Cat.No. ID-HYW-0005
Multi-Drug Rapid Test (Dipcard & Cup) with Fentanyl

Cat.No. ID-HYW-0006
Multi-Drug Rapid Test (Dipcard & Cup) without Fentanyl

Cat.No. ID-HYW-0007
Multi-Drug 2~14 Drugs Rapid Test (Dipstick & Dipcard & Cup)

Cat.No. ID-HYW-0008
Fentanyl (FYL) Rapid Test (For Prescription Use)

Cat.No. ID-HYW-0009
Fentanyl Urine Test Cassette (CLIA Waived)

Cat.No. ID-HYW-0010
Fentanyl Urine Test Cassette (Home Use)
|
There is no product in your cart. |