- Home
- Resource
- Disease Diagnosis
- Cancers
- Navigating Bladder Cancer: The Essential Role of Modern IVD in Diagnosis
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Bladder cancer stands as a significant global health challenge, characterized by its persistently high recurrence rates and substantial diagnostic complexities. This comprehensive resource focuses specifically on the evolving landscape of in vitro diagnostic (IVD) approaches for bladder cancer detection and management. We will explore in detail the complete spectrum of urine-based diagnostic methodologies, from fundamental urinalysis and traditional cytological examination to advanced molecular biomarker assays.
Bladder cancer is a malignant tumor that develops in the urothelial lining of the bladder, with smoking and occupational chemical exposures being its primary risk factors. Common symptoms include hematuria (visible or microscopic blood in urine), urinary frequency, urgency, and dysuria. While these signs may raise clinical suspicion, they are non-specific and often overlap with benign urological conditions. As one of the most prevalent cancers worldwide, bladder cancer exhibits a high recurrence rate, necessitating long-term monitoring and creating significant clinical management challenges.
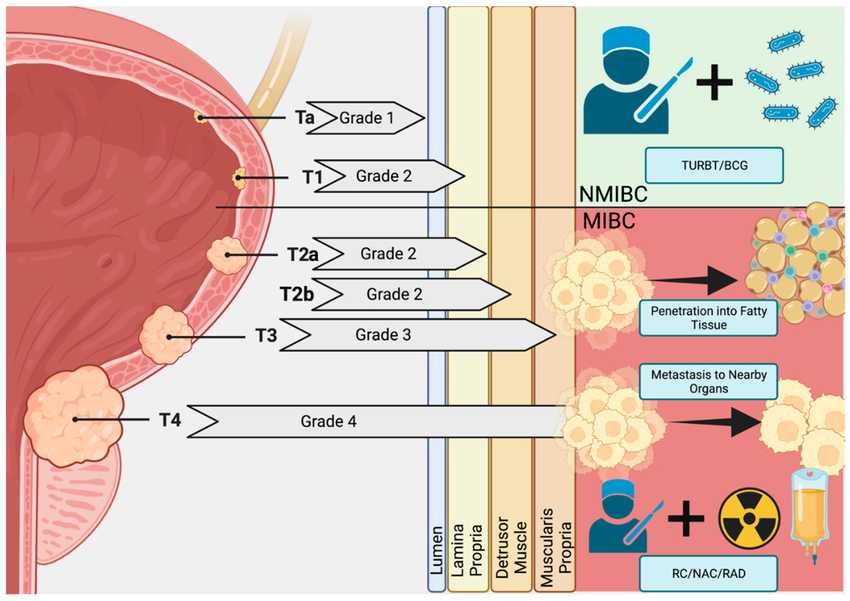 Fig.1 Bladder cancer pathogenesis. (Shi Y, et al., 2023)
Fig.1 Bladder cancer pathogenesis. (Shi Y, et al., 2023)
In the diagnosis and management of bladder cancer, in vitro diagnostics (IVD) serve as essential tools at multiple key decision points, enabling a more efficient and less invasive clinical pathway. Unlike traditional methods that rely heavily on invasive procedures, modern IVD solutions provide critical data to guide clinical decisions at every stage.
In the diagnostic evaluation of bladder cancer, core in vitro diagnostic (IVD) tests on urine samples provide essential initial data in a minimally invasive manner. These foundational tests serve as critical first-line tools for detecting abnormalities that may indicate underlying malignancy, guiding subsequent diagnostic decisions and specialized testing.
Routine Urinalysis
This fundamental test represents the most common initial screening method for urinary tract abnormalities. It primarily detects the presence of red blood cells (hematuria) - the most common presenting sign of bladder cancer - through chemical dipstick and microscopic examination. While highly sensitive for detecting blood, it lacks specificity for malignancy, as hematuria can also result from various benign conditions such as infections, stones, or trauma. Its key value lies in serving as an effective triage tool to identify patients requiring more specialized urological evaluation.
Urine Cytology Analysis
This diagnostic technique involves the microscopic examination of centrifuged urine samples to identify exfoliated abnormal or malignant urothelial cells. Its primary strength lies in its high specificity for detecting high-grade tumors and carcinoma in situ (CIS), making it particularly valuable for identifying aggressive disease variants. However, the method suffers from limited sensitivity, especially for low-grade tumors, which often shed cells that appear cytologically normal. Despite this limitation, it remains an important component in the diagnostic arsenal, particularly in monitoring patients with history of high-grade disease.
Urine biomarkers represent a significant advancement in the non-invasive detection of bladder cancer, offering improved sensitivity and objectivity compared to traditional methods. These tests analyze specific molecular indicators in urine to aid in diagnosis, surveillance, and risk stratification.

The NMP22 test detects elevated levels of nuclear matrix protein 22, a nuclear protein involved in cell division that is released during apoptosis of bladder cancer cells. This immunoassay-based test demonstrates higher sensitivity than urine cytology for detecting bladder cancer, particularly in initial diagnosis, though it can show false positives in cases of benign urological conditions. Its quantitative results provide an objective measure for clinical decision-making, making it valuable for both screening and monitoring recurrence.
BTA tests identify the presence of complement factor H and related proteins in urine, which are produced by bladder cancer cells to evade immune surveillance. Available in both qualitative (BTA stat) and quantitative (BTA TRAK) formats, these rapid immunoassays offer good sensitivity for detecting bladder cancer, especially higher-grade tumors. However, like NMP22, they may yield false positive results in patients with hematuria, urinary tract infections, or other inflammatory conditions, requiring careful interpretation in clinical context.
Alta DiagnoTech provides a comprehensive portfolio of in vitro diagnostic (IVD) solutions for bladder cancer, supporting early detection, accurate diagnosis, and effective monitoring. Our innovative products leverage advanced technologies to deliver reliable, non-invasive testing options that enhance clinical decision-making throughout the patient care pathway. If you have related needs, please feel free to contact us for more information or product support.
| Product Name | Technology | Application |
| NMP22 ELISA Kit | ELISA | Quantitative detection of nuclear matrix protein 22 in urine for bladder cancer screening and monitoring |
| BTA STAT Test | Immunochromatographic Rapid Test | Qualitative detection of bladder tumor-associated antigens in urine for point-of-care testing |
| Bladder Cancer Chromosome Abnormality Detection Kit | Fluorescence In Situ Hybridization (FISH) | Detection of chromosomal abnormalities in urine samples for diagnosis and recurrence monitoring |
| Bladder Cancer Methylation Detection Kit | Methylation Analysis (qPCR) | Detection of epigenetic markers in urine for non-invasive monitoring of recurrence |
| Bladder Cancer Gene Expression Detection Kit | Gene Expression Analysis (qPCR) | Measurement of specific mRNA biomarkers in urine for hematuria triage and early detection |
Reference
This article is for research use only. Do not use in any diagnostic or therapeutic application.
|
There is no product in your cart. |