- Home
- Resource
- Explore & Learn
- Nanobiosensor Innovations Bridging In Vitro Diagnostics to Point-of-Care Testing
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Traditional in vitro diagnostics (IVD) methods, deeply rooted in centralized laboratory settings, have long been the mainstay for disease detection. These methods, while accurate, come with significant drawbacks. The time-consuming process of sample collection, transportation to specialized labs, and subsequent analysis often results in delays, which can be critical in emergency situations or for managing rapidly progressing diseases.
Point-of-care testing (POCT) emerges as a solution to these challenges. By bringing diagnostic capabilities closer to the patient, whether in a hospital bedside, remote clinic, or even at home, POCT enables timely decision-making. However, to be truly effective, POCT requires diagnostic tools that offer high sensitivity, specificity, ease of use, and affordability. This is where nanosensors step in, leveraging the unique properties of nanomaterials to transform the landscape of diagnostics.
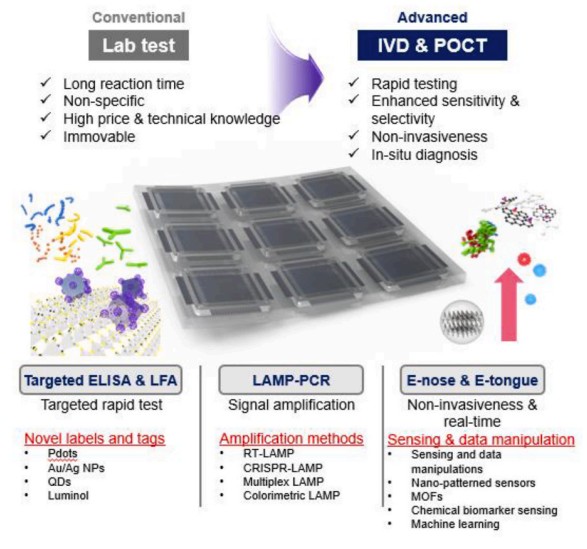 Fig.1 Implementation of IVDs in POCT. (Kim C., et al., 2024)
Fig.1 Implementation of IVDs in POCT. (Kim C., et al., 2024)
Nanomaterial Properties and Their Diagnostic Edge
Nanomaterials, with dimensions ranging from 1 to 100 nanometers, possess distinctive characteristics that make them ideal for diagnostic applications. Their high surface-area-to-volume ratio allows for enhanced interactions with target molecules. For instance, gold nanoparticles (AuNPs) can bind to biomolecules with high affinity. In a study focused on detecting a specific cancer biomarker, the use of AuNPs increased the binding efficiency by up to 30% compared to traditional methods. This property enables the detection of even minute amounts of biomarkers in biological samples, facilitating early disease detection.
Quantum dots (QDs), another type of nanomaterial, exhibit unique optical properties. They can emit fluorescent light at specific wavelengths, which can be precisely tuned depending on their size and composition. This characteristic is exploited in multiplexed diagnostic assays, where multiple biomarkers can be detected simultaneously. In an assay for infectious disease diagnosis, QDs were used to detect three different pathogens in a single sample, providing a comprehensive diagnostic result in a short time frame.
Miniaturization and Lab-on-a-Chip Technology
Nanotechnology has paved the way for the development of "lab-on-a-chip" devices. These compact systems integrate multiple laboratory functions, such as sample preparation, analysis, and result reporting, onto a single chip. Microfluidic channels, with dimensions in the nanometer to micrometer range, are used to precisely control the flow of samples and reagents. For example, a lab-on-a-chip device for blood analysis can perform multiple tests, including glucose level measurement, blood cell counting, and biomarker detection, using just a few microliters of blood. This not only reduces the sample volume required but also minimizes the need for bulky and expensive laboratory equipment.
Lateral flow assays (LFAs), commonly known for their use in home pregnancy tests and rapid COVID-19 tests, have been significantly enhanced by nanotechnology. Gold nanoparticles are widely used in LFAs due to their strong optical properties. When bound to specific antibodies or antigens, AuNPs produce a visible color change, providing a simple and intuitive readout. In a study on the detection of a cardiovascular biomarker, an LFA incorporating AuNPs achieved a detection limit of 0.5 ng/mL, which is 10 times more sensitive than traditional LFAs without nanomaterials.
Silver nanoparticles (AgNPs) also offer advantages in LFAs. Their catalytic properties can be used to enhance the color development, further improving the sensitivity of the assay. A study demonstrated that by using AgNPs in an LFA for detecting a viral antigen, the detection time was reduced from 15 minutes to 8 minutes, while maintaining high sensitivity.
Quantum dots enable multiplexed detection in immunodiagnostics. Each QD can be functionalized with a different antibody or antigen, allowing for the simultaneous detection of multiple targets. In a clinical study for autoimmune disease diagnosis, a QD-based LFA was able to detect five different autoantibodies in a single sample. The fluorescent signals from the QDs were analyzed using a fluorescence reader, providing accurate and quantitative results for each biomarker. This multiplexing capability not only saves time but also reduces the sample volume required, making it more patient-friendly.

Isothermal Amplification and Nanoparticle Integration
Loop-mediated isothermal amplification (LAMP) is a nucleic acid amplification technique that operates at a constant temperature, making it suitable for point-of-care applications. Nanoparticles can be integrated with LAMP to improve its performance. Magnetic nanoparticles (MNPs) are often used for nucleic acid extraction. By coating MNPs with specific oligonucleotides that bind to the target nucleic acid, the target can be selectively captured from a complex biological sample. After extraction, the MNPs can be easily separated using a magnetic field, providing a purified sample for subsequent LAMP amplification.
In a study on detecting a viral nucleic acid, the use of MNPs in combination with LAMP reduced the background noise by 50%, resulting in a more accurate and sensitive detection. Additionally, gold nanoparticles can be used to enhance the amplification efficiency of LAMP. They interact with the DNA polymerase enzyme used in the LAMP reaction, improving its activity and reducing the amplification time.

CRISPR-Cas Systems and Nanosensors
The CRISPR-Cas system, known for its gene-editing capabilities, has also found applications in diagnostics. When combined with nanosensors, it offers high specificity and sensitivity for nucleic acid detection. Nanosensors can be used to detect the cleavage products generated by the CRISPR-Cas system. For example, in a diagnostic assay for a genetic disorder, a nanowire-based sensor was used to detect the presence of a mutated DNA sequence. The nanowire was functionalized with probes that specifically bind to the cleavage products of the CRISPR-Cas reaction. The change in the electrical properties of the nanowire upon binding of the products was measured, providing a rapid and accurate diagnostic result.
Exhaled breath contains volatile organic compounds (VOCs) that can serve as biomarkers for various diseases. Nanosensors are being developed to create artificial "noses" (e-noses) for breath analysis. Metal oxide semiconductor (MOS) sensors with nanopatterned surfaces are highly sensitive to VOCs. The nanopatterns increase the surface area of the sensor, allowing for more efficient adsorption of VOCs. In a study on lung cancer detection, an e-nose based on MOS sensors was able to distinguish between breath samples of lung cancer patients and healthy individuals with an accuracy of 85%.
Metal-organic frameworks (MOFs) are another class of nanomaterials used in e-noses. MOFs have a porous structure that can selectively adsorb specific VOCs. When combined with other nanomaterials, such as graphene, MOFs can create highly sensitive and selective sensors. In a proof-of-concept study, an MOF-based sensor was able to detect a specific VOC associated with diabetes at concentrations as low as 10 parts per billion.
Nanofluidic sensors are designed to analyze body fluids such as saliva, sweat, and urine. These sensors can detect chemical biomarkers with high sensitivity. For example, in a sweat analysis system, nanofluidic channels are used to collect and analyze sweat samples. The channels are functionalized with receptors that specifically bind to target biomarkers, such as electrolytes or metabolites. The change in the electrical or optical properties of the sensor upon binding of the biomarkers is measured, providing real-time information about the physiological state of the individual. This technology has the potential to be integrated into wearable devices for continuous health monitoring.
Nanosensor technologies have redefined the landscape of in vitro diagnostics, bridging the gap between laboratory precision and point-of-care accessibility. By harnessing the unique properties of nanomaterials—enhanced sensitivity, multiplexing capabilities, and miniaturization—these tools enable rapid, accurate detection of biomarkers across immunological, molecular, and chemical diagnostic modalities. From LFAs for infectious diseases to SERS-based assays for early cancer detection, and from LAMP-integrated LoCs to e-noses for non-invasive monitoring, nanosensors are driving a shift toward personalized, patient-centric healthcare. As research continues to refine their performance—improving stability, reducing costs, and expanding multiplexing, nanosensors will undoubtedly play a central role in global health, enabling timely interventions, preventing disease spread, and ultimately improving patient outcomes.
If you have related needs, please feel free to contact us for more information or product support.
Reference
This article is for research use only. Do not use in any diagnostic or therapeutic application.

Cat.No. GP-DQL-00203
Rotavirus Antigen Group A and Adenovirus Antigen Rapid Test Kit (Colloidal Gold)

Cat.No. GP-DQL-00206
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Card Style

Cat.No. GP-DQL-00207
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. GP-DQL-00211
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. GP-DQL-00212
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. IP-00189
Influenza A Rapid Assay Kit

Cat.No. GH-DQL-00200
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold)
Cat.No. GH-DQL-00201
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00202
Luteinizing Hormone Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00208
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold), Strip Style
Cat.No. GH-DQL-00209
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit(Colloidal Gold), Strip Style

Cat.No. GH-DQL-00210
Luteinizing Hormone Rapid Test Kit (Colloidal Gold), Strip Style
Cat.No. IH-HYW-0001
hCG Pregnancy Test Strip
Cat.No. IH-HYW-0002
hCG Pregnancy Test Cassette
Cat.No. IH-HYW-0003
hCG Pregnancy Test Midstream

Cat.No. GD-QCY-0001
Cocaine (COC) Rapid Test Kit

Cat.No. GD-QCY-0002
Marijuana (THC) Rapid Test Kit
Cat.No. GD-QCY-0003
Morphine (MOP300) Rapid Test Kit
Cat.No. GD-QCY-0004
Methamphetamine (MET) Rapid Test Kit
Cat.No. GD-QCY-0005
Methylenedioxymethamphetamine ecstasy (MDMA) Rapid Test Kit
Cat.No. GD-QCY-0006
Amphetamine (AMP) Rapid Test Kit
Cat.No. GD-QCY-0007
Barbiturates (BAR) Rapid Test Kit

Cat.No. GD-QCY-0008
Benzodiazepines (BZO) Rapid Test Kit
Cat.No. GD-QCY-0009
Methadone (MTD) Rapid Test Kit
Cat.No. GD-QCY-0011
Opiate (OPI) Rapid Test Kit
Cat.No. ID-HYW-0002
Multi-Drug Test L-Cup, (5-16 Para)
Cat.No. ID-HYW-0005
Multi-Drug Rapid Test (Dipcard & Cup) with Fentanyl
Cat.No. ID-HYW-0006
Multi-Drug Rapid Test (Dipcard & Cup) without Fentanyl
Cat.No. ID-HYW-0007
Multi-Drug 2~14 Drugs Rapid Test (Dipstick & Dipcard & Cup)
Cat.No. ID-HYW-0008
Fentanyl (FYL) Rapid Test (For Prescription Use)
Cat.No. ID-HYW-0009
Fentanyl Urine Test Cassette (CLIA Waived)
Cat.No. ID-HYW-0010
Fentanyl Urine Test Cassette (Home Use)
|
There is no product in your cart. |