- Home
- Resource
- Disease Diagnosis
- Cardiovascular Diseases
- Myocarditis Diagnosis: Integrating Biomarkers, Imaging, and Gold-Standard Techniques
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Myocarditis is an inflammatory disease of the heart muscle with a challenging and often non-specific clinical presentation. This resource provides a comprehensive, step-by-step guide to its modern diagnostic workup, detailing how to effectively integrate key biomarkers, advanced cardiac imaging, and gold-standard pathological techniques to achieve a confident diagnosis.
Myocarditis is an inflammatory condition of the heart muscle (myocardium) characterized by a diverse range of potential causes, most commonly viral infections. This inflammation can directly damage cardiac myocytes and disrupt electrical conduction, leading to a highly variable clinical presentation—from asymptomatic cases and chest pain mimicking a heart attack to acute heart failure, arrhythmias, and sudden cardiac death. The diagnosis poses a significant clinical challenge due to its non-specific symptoms, necessitating a high index of suspicion and a multimodal diagnostic approach that strategically integrates cardiac biomarkers, advanced imaging, and, in select cases, endomyocardial biopsy for definitive confirmation.
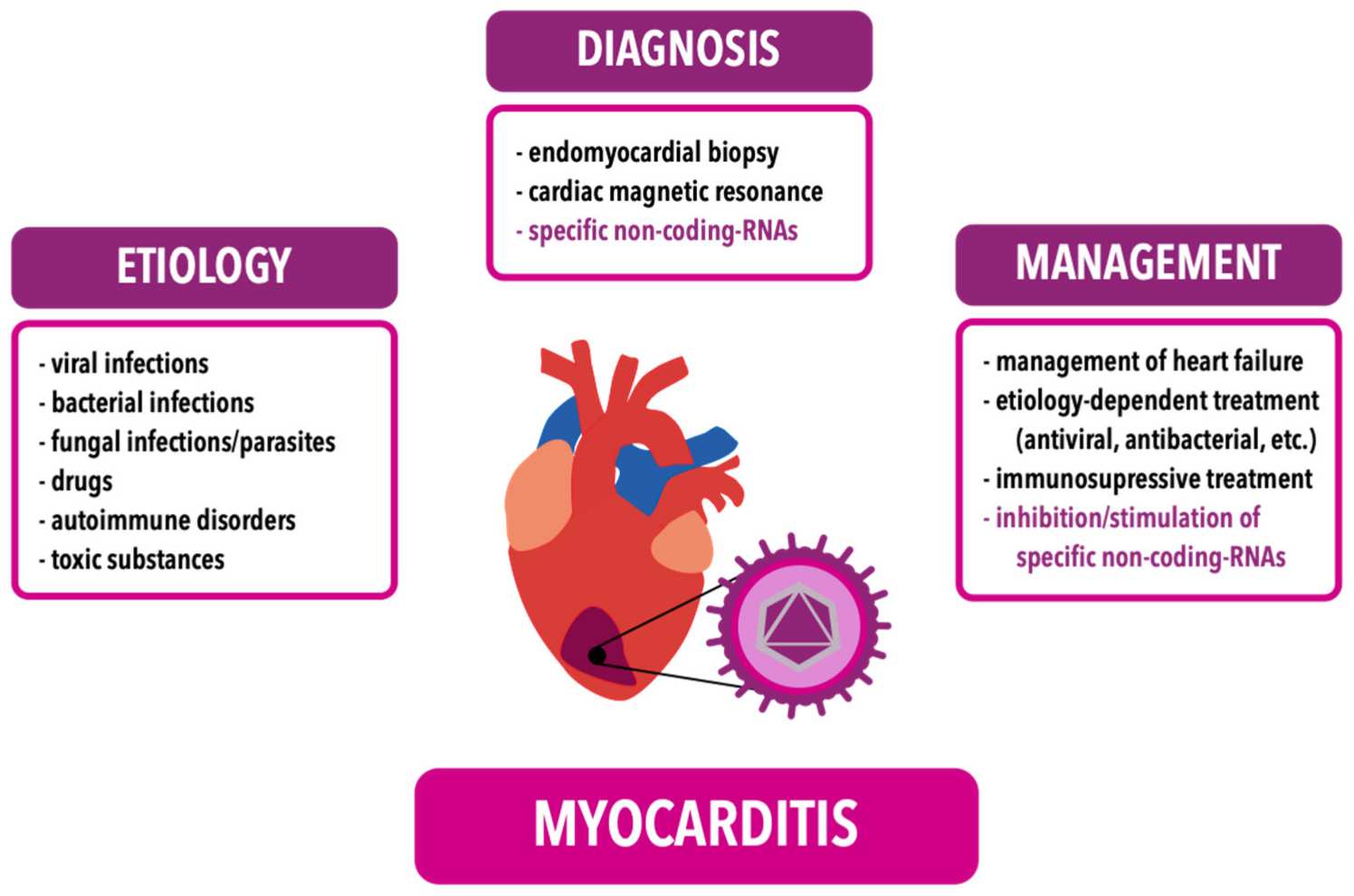 Fig.1 Etiology, diagnosis and management of myocarditis. (Młynarska E, et al., 2024)
Fig.1 Etiology, diagnosis and management of myocarditis. (Młynarska E, et al., 2024)
The diagnosis of myocarditis relies heavily on a panel of serum biomarkers that provide critical, albeit often non-specific, insights into the underlying cardiac pathology. These biomarkers serve as accessible tools to detect myocyte injury, assess hemodynamic stress, and gauge the systemic inflammatory response. The core panel integrates three key aspects: cardiac troponin for cellular damage, natriuretic peptides for ventricular strain, and general inflammatory markers.
Cardiac Troponin (I/T)
Cardiac troponin (I/T) is the cornerstone biomarker for detecting myocyte injury and necrosis. Released into the bloodstream upon damage to the cardiac muscle cells, it exhibits high sensitivity for acute myocarditis. While elevated troponin levels strongly indicate active cardiac injury, they are not specific to myocarditis and can be seen in other conditions like acute coronary syndromes.
Natriuretic Peptides (BNP/NT-proBNP)
Natriuretic peptides (BNP/NT-proBNP) are hormones released by the ventricles in response to increased wall stress and volume overload. They are crucial indicators of the functional consequence of myocarditis, often correlating with the development of heart failure. Their levels are valuable for assessing the hemodynamic impact of the disease and guiding heart failure management, though they too can be elevated in any cause of cardiac strain.
Inflammatory Markers (CRP & ESR)
Inflammatory markers (CRP & ESR) are non-specific acute-phase reactants that rise in response to systemic inflammation. In the context of myocarditis, they can support the presence of an active inflammatory state, which may be linked to the underlying cause. However, their significant limitation is their lack of cardiac specificity, as elevations can result from any concurrent infection, autoimmune disorder, or other inflammatory condition.
While biomarkers signal that injury is occurring, advanced imaging, particularly cardiac magnetic resonance (CMR), allows us to visualize the injury directly within the heart muscle, making it the cornerstone of non-invasive diagnosis. CMR provides a comprehensive tissue characterization by utilizing multiple sequences to detect the key hallmarks of myocarditis: myocardial edema, hyperemia (increased blood flow), and necrosis or fibrosis. This multi-parametric approach is formalized in the lake louise criteria (LLC), which established CMR as a primary tool for confirming the diagnosis without the need for a biopsy.

T2-weighted imaging is used to detect myocardial edema, a key sign of active inflammation. By measuring the water content within the tissue, this sequence can identify areas of the heart muscle that are swollen and inflamed, providing a clear picture of the disease's acute activity.

Early gadolinium enhancement (EGE) indicates hyperemia and capillary leak. Following an injection of a gadolinium-based contrast agent, rapid imaging assesses how quickly the contrast pools into the inflamed tissue. Increased enhancement suggests heightened blood flow and leaky capillaries, which are characteristic of the acute inflammatory response in myocarditis.

Late gadolinium enhancement (LGE) reveals areas of myocardial necrosis and fibrosis. Taken several minutes after contrast injection, this sequence highlights regions where the contrast agent has accumulated in the extracellular space of irreversibly damaged or scarred tissue. The typical pattern for myocarditis is a patchy, mid-myocardial or subepicardial distribution, which helps distinguish it from the subendocardial scarring of a myocardial infarction.
Endomyocardial biopsy (EMB) remains the diagnostic gold standard for myocarditis as it provides definitive histopathological confirmation through direct tissue analysis. This invasive procedure involves catheter-based retrieval of small myocardial tissue samples, which are then examined using histology, immunohistochemistry, and polymerase chain reaction (PCR) to identify characteristic inflammatory cell infiltrates, myocyte necrosis, and specific viral genomes. However, its use is strategically reserved for severe or complex cases—such as rapidly deteriorating heart failure or life-threatening arrhythmias—due to inherent risks of procedural complications and the potential for sampling error, given the frequently patchy nature of myocardial inflammation.
Alta DiagnoTech provides a comprehensive portfolio of diagnostic and research solutions for myocarditis, empowering clinicians and researchers to achieve precise and timely insights. Our integrated offerings span from robust in vitro diagnostics (IVD) for routine clinical assessment to cutting-edge research tools that drive the discovery of novel biomarkers and pathogenic mechanisms, supporting every stage of patient management and scientific inquiry. If you have related needs, please feel free to contact us for more information or product support.
| Product Name | Technology | Application |
| High-Sensitivity Cardiac Troponin I (cTnI) Assay | Chemiluminescence Immunoassay (CLIA) | Quantitative measurement of cTnI in serum/plasma for the assessment of myocardial injury; IVD |
| NT-proBNP Electrochemiluminescence Assay | Electrochemiluminescence Immunoassay (ECLIA) | Quantitative measurement of NT-proBNP in serum/plasma to aid in heart failure evaluation; IVD |
| sST2 ELISA Kit | Enzyme-Linked Immunosorbent Assay (ELISA) | Quantitative detection of soluble ST2 in human serum for research on myocardial stress and fibrosis; Research Use Only (RUO) |
| Cardiac Autoantibody Panel | Line Immunoassay / Multiplex Bead Array | Simultaneous detection of multiple circulating cardiac autoantibodies (e.g., anti-β1, anti-M2) for research into autoimmune myocarditis; RUO |
| Myocarditis-Associated Viral PCR Panel | Quantitative Polymerase Chain Reaction (qPCR) | Detection and quantification of common viral genomes (e.g., Parvovirus B19, HHV-6, Coxsackievirus) in plasma or tissue samples; RUO |
| Cardiac Myosin Heavy Chain ELISA | Enzyme-Linked Immunosorbent Assay (ELISA) | Quantitative analysis of cardiac-specific myosin heavy chain in serum as a potential research biomarker for severe myocyte damage; RUO |
Reference
This article is for research use only. Do not use in any diagnostic or therapeutic application.
|
There is no product in your cart. |