- Home
- Resource
- Explore & Learn
- Molecular Allergology as a Precision-Medicine Toolkit: Diagnosing, Stratifying, and Monitoring Allergic Patients
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Allergic diseases are a rising global health concern, affecting nearly 30% of the world's population, with variations across age groups and geographic regions. The complexity of allergenic sources—ranging from pollens and foods to venoms and molds—combined with individual immune variability, has long hindered precise diagnosis and targeted management. However, the advent of molecular allergology has introduced an unprecedented shift toward precision diagnostics by identifying specific allergenic proteins, or molecular allergens (MAs), responsible for IgE-mediated hypersensitivity reactions.
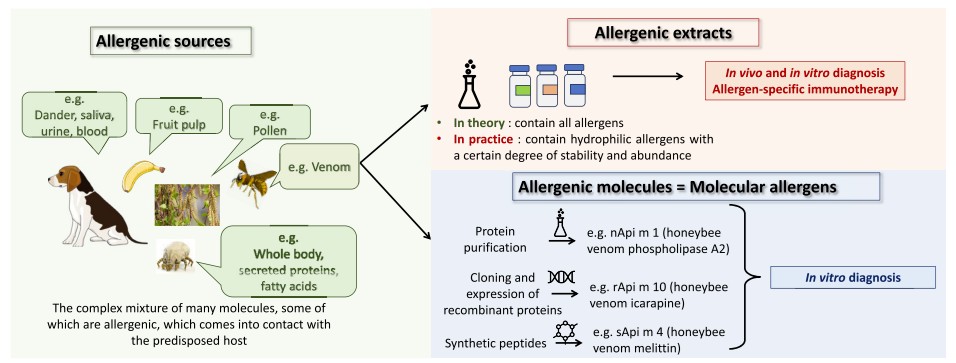 Fig.1 Concepts of allergenic source, allergen extract, molecular allergen, and IgE epitope. (Giusti D., et al., 2024)
Fig.1 Concepts of allergenic source, allergen extract, molecular allergen, and IgE epitope. (Giusti D., et al., 2024)
IgE-mediated allergic diseases progress through two phases: sensitization and effector response.
During the sensitization phase, allergens cross epithelial barriers, are taken up by dendritic cells, and are presented to naïve T cells, which differentiate into Th2 cells under the influence of interleukin-4 (IL-4). These cells stimulate B-cell isotype switching to produce allergen-specific IgE antibodies, which bind FcεRI receptors on mast cells and basophils, priming them for rapid activation.
Upon re-exposure to the allergen, cross-linking of IgE-FcεRI complexes on mast cells triggers immediate degranulation, releasing histamine, proteases, and other proinflammatory mediators. A late-phase reaction follows, characterized by leukocyte recruitment and cytokine-driven inflammation, contributing to chronic allergic disease progression.
Traditional allergy testing has relied on allergenic extracts, complex mixtures derived from pollen, foods, or venom, with significant variability in protein content and diagnostic reliability. Many proteins in these extracts are denatured, lost, or underrepresented, such as oleosins in peanuts or peamaclein Pru p 7 in peach, leading to false negatives or inaccurate results.
Molecular allergology overcomes these limitations by using purified, recombinant, or synthetic MAs, allowing:
MA testing is exclusively available for in vitro diagnostics (IVD), using fluorescence, chemiluminescence, or microarray platforms.
Molecular allergens are classified into over 180 biochemical families, each associated with distinct sensitization profiles, exposure routes, and clinical outcomes.
Table 1. Selected Allergen Families and Clinical Correlates.
| Family | Source | Exposure | Clinical Relevance |
| PR-10 | Birch, apple, hazelnut | Food, Airborne | Oral allergy syndrome |
| nsLTP | Peach, olive, peanut | Food | Systemic reactions, severe anaphylaxis |
| Profilins | Pollens, fruits | Food, Airborne | Panallergens, cross-reactivity |
| Lipocalins | Cat, dog, milk | Airborne, Food | Asthma, respiratory allergy |
| Tropomyosins | Mites, shrimp, cockroaches | Food, Airborne | Cross-reactive food and respiratory allergies |
| Storage Proteins | Peanut, sesame, tree nuts | Food | Severe reactions, diagnostic markers |
Marker allergens are uniquely expressed proteins within a specific allergenic source and serve as diagnostic signatures of genuine sensitization.
For instance:
These MAs are critical for initiating allergen-specific immunotherapy (AIT), dietary avoidance plans, and personalized risk mitigation.
Cross-reactivity arises when IgE antibodies target structurally similar epitopes across unrelated allergenic sources. Panallergen families such as profilins, tropomyosins, and polcalcins are frequently implicated.
Clinical scenarios include:
Understanding cross-reactivity is vital to avoid overdiagnosis and to refine AIT eligibility.
Certain MAs predict severe allergic reactions and persistent disease:
Sensitization to Fel d 1, Fel d 4, and Can f 1/2 correlates with asthma phenotypes, especially those with type 2 inflammation. Elevated IgE to lipocalins further predicts refractory or persistent symptoms.
Early detection of Phl p 5 (grass pollen) or Der p 1 (dust mites) sensitization in preschoolers predicts the onset of adolescent asthma, guiding preventive strategies and immunotherapy timing.
Image analysis on page 9 illustrates these two workflows, highlighting the advantage of simultaneous profiling in multiplex formats and quantitative rigor in singleplex systems.
Despite its strengths, molecular allergology still faces hurdles:
Cross-disciplinary collaboration, involving immunologists, laboratory experts, and allergists, is critical to fully integrate molecular testing into routine care. Europe is spearheading interdisciplinary allergy units to close this gap and expand the use of MA-based diagnostics.
Molecular allergology has transformed allergy diagnostics from a generalized, symptom-based discipline into a molecularly guided, stratified, and personalized specialty. By decoding the IgE response at the molecular level, clinicians can distinguish between genuine and cross-reactive sensitizations, stratify patients by risk, and guide tailored therapy, including AIT.
As the field matures and becomes more accessible, molecular allergen testing is poised to become the gold standard in allergy diagnostics, shaping the next era of precision immunology and personalized medicine.
If you have related needs, please feel free to contact us for more information or product support.
Reference
This article is for research use only. Do not use in any diagnostic or therapeutic application.

Cat.No. GP-DQL-00203
Rotavirus Antigen Group A and Adenovirus Antigen Rapid Test Kit (Colloidal Gold)

Cat.No. GP-DQL-00206
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Card Style

Cat.No. GP-DQL-00207
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. GP-DQL-00211
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. GP-DQL-00212
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. IP-00189
Influenza A Rapid Assay Kit

Cat.No. GH-DQL-00200
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00201
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00202
Luteinizing Hormone Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00208
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. GH-DQL-00209
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit(Colloidal Gold), Strip Style

Cat.No. GH-DQL-00210
Luteinizing Hormone Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. IH-HYW-0001
hCG Pregnancy Test Strip

Cat.No. IH-HYW-0002
hCG Pregnancy Test Cassette

Cat.No. IH-HYW-0003
hCG Pregnancy Test Midstream

Cat.No. GD-QCY-0001
Cocaine (COC) Rapid Test Kit

Cat.No. GD-QCY-0002
Marijuana (THC) Rapid Test Kit

Cat.No. GD-QCY-0003
Morphine (MOP300) Rapid Test Kit

Cat.No. GD-QCY-0004
Methamphetamine (MET) Rapid Test Kit

Cat.No. GD-QCY-0005
Methylenedioxymethamphetamine ecstasy (MDMA) Rapid Test Kit

Cat.No. GD-QCY-0006
Amphetamine (AMP) Rapid Test Kit

Cat.No. GD-QCY-0007
Barbiturates (BAR) Rapid Test Kit

Cat.No. GD-QCY-0008
Benzodiazepines (BZO) Rapid Test Kit

Cat.No. GD-QCY-0009
Methadone (MTD) Rapid Test Kit

Cat.No. GD-QCY-0011
Opiate (OPI) Rapid Test Kit

Cat.No. ID-HYW-0002
Multi-Drug Test L-Cup, (5-16 Para)

Cat.No. ID-HYW-0005
Multi-Drug Rapid Test (Dipcard & Cup) with Fentanyl

Cat.No. ID-HYW-0006
Multi-Drug Rapid Test (Dipcard & Cup) without Fentanyl

Cat.No. ID-HYW-0007
Multi-Drug 2~14 Drugs Rapid Test (Dipstick & Dipcard & Cup)

Cat.No. ID-HYW-0008
Fentanyl (FYL) Rapid Test (For Prescription Use)

Cat.No. ID-HYW-0009
Fentanyl Urine Test Cassette (CLIA Waived)

Cat.No. ID-HYW-0010
Fentanyl Urine Test Cassette (Home Use)
|
There is no product in your cart. |