- Home
- Resource
- Explore & Learn
- Microbiome Biomarkers for Periodontitis: A Systematic Review and Meta-Analysis of Diagnostic Performance
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Periodontitis, a chronic inflammatory disease, poses a significant global health challenge. With projections indicating that severe cases could surge by 44% by 2050, affecting over 1 billion individuals worldwide, the urgency of accurate and early diagnosis cannot be overstated. Traditional diagnostic methods, primarily relying on clinical parameters such as probing pocket depth and radiographic assessment, have inherent limitations. These methods predominantly focus on evaluating the structural damage caused by the disease rather than detecting the active presence of pathogenic bacteria. For instance, pocket probing measures the depth of the space between the gum and tooth, but it fails to provide real-time information about the ongoing infection process, often resulting in delayed diagnosis when substantial tissue destruction has already occurred.
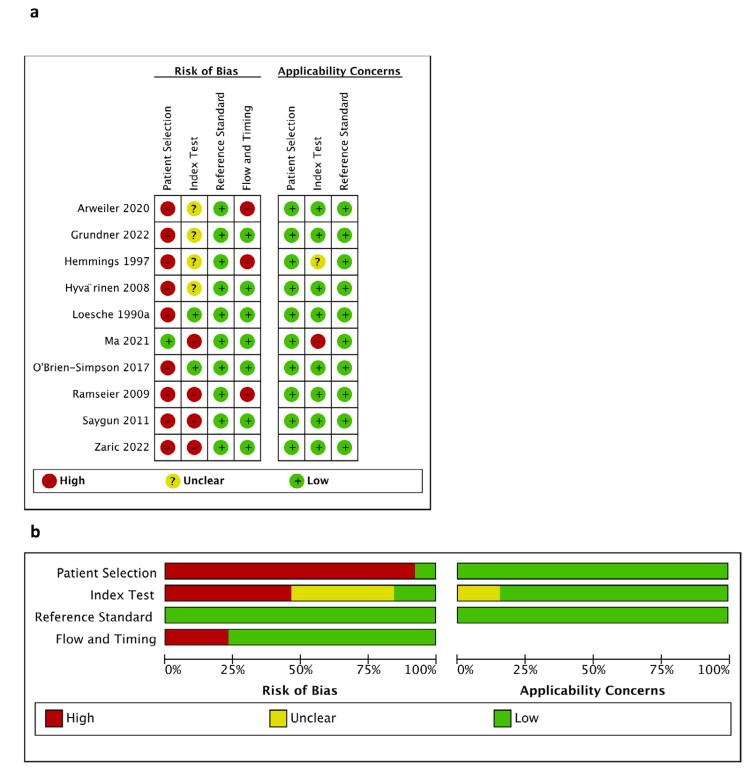 Fig.1 (a) Risk of bias and applicability concerns evaluation for the reviewed studies (PRISMA Framework). (b) Summary of risk of bias and applicability issues (PRISMA). (Dong A., et al., 2025)
Fig.1 (a) Risk of bias and applicability concerns evaluation for the reviewed studies (PRISMA Framework). (b) Summary of risk of bias and applicability issues (PRISMA). (Dong A., et al., 2025)
The oral cavity harbors a complex and diverse microbiome, consisting of hundreds of bacterial species. In a healthy state, this microbiome maintains a delicate balance. However, in periodontitis, there is a dysbiosis, a shift in the microbial community composition, where pathogenic bacteria gain dominance. Key periodontal pathogens, such as Porphyromonas gingivalis, Tannerella forsythia, and Treponema denticola, play crucial roles in the disease process. Porphyromonas gingivalis, for example, produces a variety of virulence factors, including proteases that degrade host tissues and evade the immune response, facilitating the progression of periodontitis. Understanding the composition and dynamics of the oral microbiome is thus essential for developing more effective diagnostic strategies.
Saliva offers a convenient and non-invasive source for biomarker discovery in periodontitis diagnosis. Several bacterial species have emerged as promising biomarkers in saliva samples. Porphyromonas gingivalis has demonstrated remarkable diagnostic potential, with a sensitivity of 89.2% and a specificity of 94.6%, translating to an area under the curve (AUC) value above 0.80, which is indicative of excellent diagnostic accuracy. Similarly, Tannerella forsythia shows comparable sensitivity (89.2%) and a high specificity of 86.5%, also achieving a high AUC. Prevotella intermedia has an 86.5% sensitivity and 83.8% specificity, contributing to the pool of salivary biomarkers.

Combining multiple bacterial biomarkers in saliva can further enhance diagnostic performance. For example, a study that investigated 11 subgingival plaque-specific bacteria in saliva achieved a sensitivity of 90.5%, highlighting the synergistic effect of multiple biomarkers. However, not all salivary biomarkers perform equally well; salivary endotoxin, for instance, has shown relatively lower sensitivity at 69%, indicating the need for careful biomarker selection.
Subgingival plaque, located in the periodontal pocket, is the epicenter of periodontal infection. Biomarkers derived from this site tend to exhibit higher diagnostic accuracy compared to those in saliva. Endotoxin activity, which measures the presence of bacterial toxins in subgingival plaque, has shown outstanding performance with a sensitivity of 90.6% and a specificity of 87.9%, resulting in an AUC of 0.93. Combinations of bacterial biomarkers also excel in subgingival plaque analysis. Testing for a panel of 5 bacterial species achieved a sensitivity of 85.1% and a perfect specificity of 100%, with an AUC of 0.88.
Treponema denticola, often associated with advanced periodontitis, shows 82% sensitivity and 83% specificity in subgingival samples. Chairside tests (CSTs) that detect multiple periodontal pathogens simultaneously have proven to be highly effective, with a sensitivity of 85% and a specificity of 100%, making them a practical tool for immediate in-office diagnosis.
| Biomarker Type | Sample Source | Sensitivity | Specificity | AUC |
| Porphyromonas gingivalis | Saliva | 89.2% | 94.6% | 0.92 |
| Endotoxin activity | Subgingival plaque | 90.6% | 87.9% | 0.93 |
| 5-bacteria panel | Subgingival plaque | 85.1% | 100% | 0.88 |
| Tannerella forsythia | Saliva | 89.2% | 86.5% | 0.88 |
This table illustrates the varying performance of different biomarkers. While subgingival plaque biomarkers generally exhibit higher accuracy, saliva-based biomarkers offer the advantage of non-invasiveness, making them suitable for initial screening. Porphyromonas gingivalis stands out as a versatile biomarker with good performance in both sample types.
The integration of oral microbiome biomarkers into clinical practice has the potential to revolutionize periodontitis diagnosis. Dentists could perform quick and accurate tests during routine check-ups, enabling early detection of the disease, even in patients with minimal clinical symptoms. For high-risk patients, such as smokers or those with diabetes, biomarker-based diagnostics can identify subclinical infections, allowing for timely intervention and prevention of disease progression.
However, several challenges need to be addressed for widespread implementation. Standardization of sample collection, processing, and biomarker analysis across different laboratories is crucial. Future research should focus on validating biomarkers in larger, diverse populations, exploring novel biomarker combinations, and developing user-friendly point-of-care testing platforms. Additionally, investigating the role of other components of the oral microbiome, such as bacteriophages and metabolites, could further enhance our understanding of periodontitis pathogenesis and diagnosis.
In conclusion, oral microbiome biomarkers hold great promise in transforming periodontitis diagnosis from a reactive approach based on tissue damage assessment to a proactive, infection-focused strategy. With continued research and development, these biomarkers could become an integral part of routine periodontal care, improving patient outcomes and reducing the global burden of periodontitis.
If you have related needs, please feel free to contact us for more information or product support.
Reference
This article is for research use only. Do not use in any diagnostic or therapeutic application.

Cat.No. GP-DQL-00203
Rotavirus Antigen Group A and Adenovirus Antigen Rapid Test Kit (Colloidal Gold)

Cat.No. GP-DQL-00206
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Card Style

Cat.No. GP-DQL-00207
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. GP-DQL-00211
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. GP-DQL-00212
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. IP-00189
Influenza A Rapid Assay Kit

Cat.No. GH-DQL-00200
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00201
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00202
Luteinizing Hormone Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00208
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. GH-DQL-00209
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit(Colloidal Gold), Strip Style

Cat.No. GH-DQL-00210
Luteinizing Hormone Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. IH-HYW-0001
hCG Pregnancy Test Strip

Cat.No. IH-HYW-0002
hCG Pregnancy Test Cassette

Cat.No. IH-HYW-0003
hCG Pregnancy Test Midstream

Cat.No. GD-QCY-0001
Cocaine (COC) Rapid Test Kit

Cat.No. GD-QCY-0002
Marijuana (THC) Rapid Test Kit

Cat.No. GD-QCY-0003
Morphine (MOP300) Rapid Test Kit

Cat.No. GD-QCY-0004
Methamphetamine (MET) Rapid Test Kit

Cat.No. GD-QCY-0005
Methylenedioxymethamphetamine ecstasy (MDMA) Rapid Test Kit

Cat.No. GD-QCY-0006
Amphetamine (AMP) Rapid Test Kit

Cat.No. GD-QCY-0007
Barbiturates (BAR) Rapid Test Kit

Cat.No. GD-QCY-0008
Benzodiazepines (BZO) Rapid Test Kit

Cat.No. GD-QCY-0009
Methadone (MTD) Rapid Test Kit

Cat.No. GD-QCY-0011
Opiate (OPI) Rapid Test Kit

Cat.No. ID-HYW-0002
Multi-Drug Test L-Cup, (5-16 Para)

Cat.No. ID-HYW-0005
Multi-Drug Rapid Test (Dipcard & Cup) with Fentanyl

Cat.No. ID-HYW-0006
Multi-Drug Rapid Test (Dipcard & Cup) without Fentanyl

Cat.No. ID-HYW-0007
Multi-Drug 2~14 Drugs Rapid Test (Dipstick & Dipcard & Cup)

Cat.No. ID-HYW-0008
Fentanyl (FYL) Rapid Test (For Prescription Use)

Cat.No. ID-HYW-0009
Fentanyl Urine Test Cassette (CLIA Waived)

Cat.No. ID-HYW-0010
Fentanyl Urine Test Cassette (Home Use)
|
There is no product in your cart. |