- Home
- Resource
- Explore & Learn
- Microbial Extracellular Vesicles and AI: Revolutionizing Personalized Diagnostics
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Microbial Extracellular Vesicles (MEVs) represent a previously underappreciated communication system between the human microbiome and host cells. These nano-sized structures, typically ranging from 30 to 1000 nm in diameter, are released by various microorganisms, including bacteria, fungi, and archaea. MEVs carry a diverse cargo of proteins, nucleic acids, lipids, and metabolites, enabling them to act as key mediators of interkingdom signaling.
Two primary types of MEVs exist: ectosomes, which bud directly from the microbial plasma membrane, and apoptotic bodies, released during microbial cell death. Both types possess the unique ability to traverse biological barriers, including the mucosal barrier and even the blood-brain barrier. This remarkable property allows MEVs to circulate systemically, influencing distant tissues and organs. For instance, experimental evidence demonstrates that MEVs derived from Aggregatibacter actinomycetemcomitans can cross the blood-brain barrier and be detected in brain tissues within 24 hours of systemic administration.
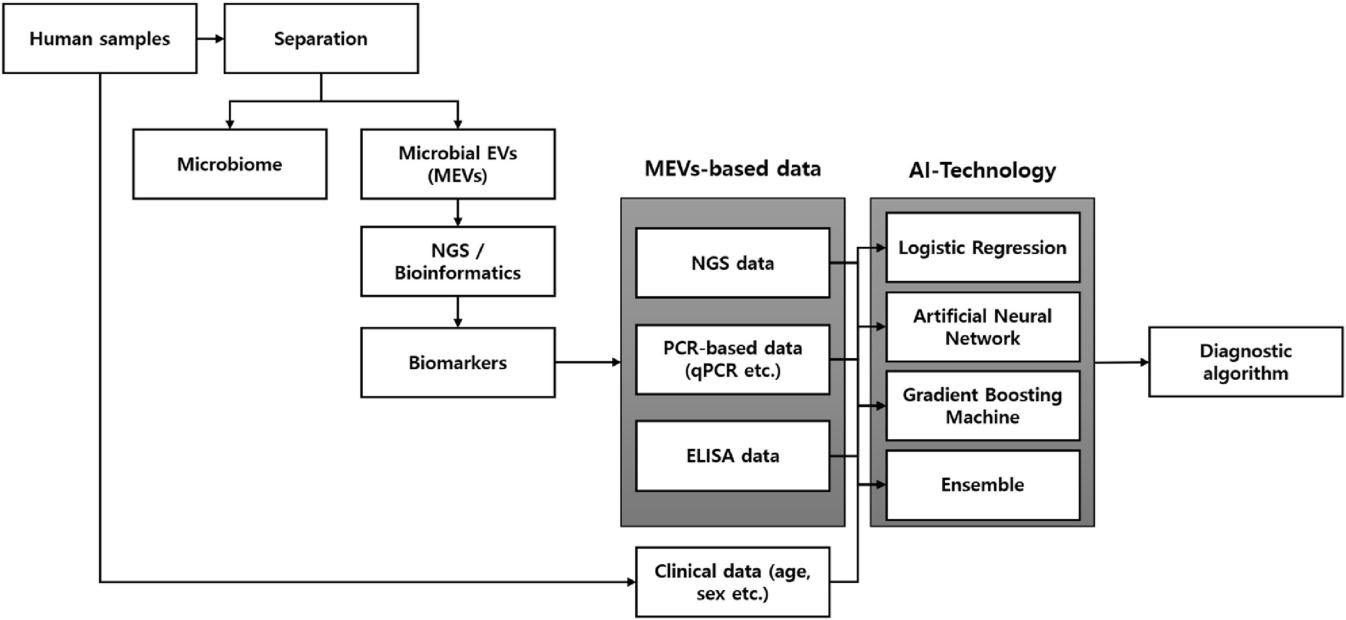 Fig.1 Flow chart of the development of an algorithm utilizing biomarkers based on microbial extracellular vesicles as in vitro diagnostics. (Yang J., 2024)
Fig.1 Flow chart of the development of an algorithm utilizing biomarkers based on microbial extracellular vesicles as in vitro diagnostics. (Yang J., 2024)
MEVs interact with host cells through three primary mechanisms: endocytosis, internalization via lipid rafts, and direct membrane fusion. These interactions trigger diverse cellular responses, from immune modulation to metabolic regulation, making MEVs critical players in both health maintenance and disease pathogenesis. Their ability to carry specific molecular signatures of their microbial origin and the host's physiological state positions them as ideal candidates for diagnostic applications.
The journey from biological sample to diagnostic insight begins with robust MEV isolation techniques. While ultracentrifugation remains the gold standard, protocols have been refined for different sample types to maximize yield and purity. For urine samples, centrifugation at 10,000×g for 10 minutes at 4°C followed by 0.22 μm filtration effectively removes contaminants. Serum samples undergo similar processing after dilution in phosphate-buffered saline (PBS), while fecal samples require initial dilution in PBS at a 1:10 ratio and 24-hour storage at 4°C before centrifugation.
Once isolated, MEVs undergo comprehensive characterization using metagenomic approaches. Next-Generation Sequencing (NGS) technologies, particularly those targeting the 16S rRNA gene, provide detailed taxonomic profiles of the microbial origin of these vesicles. Recent shifts toward analyzing the V3-V4 region of the 16S rRNA gene have improved taxonomic resolution, while shotgun metagenomics enables species-level identification and functional pathway analysis.

Bioinformatics pipelines play a crucial role in transforming raw sequencing data into meaningful insights. After quality filtering to remove reads with Phred scores below 20 and eliminating chimeric sequences, advanced tools like DADA2 process the data to identify Amplicon Sequence Variants (ASVs). These ASVs, which represent unique microbial fingerprints, are then taxonomically classified using databases such as SILVA, Greengenes, or EzTaxon. Functional analysis using KEGG and BLAST further elucidates the biological pathways associated with detected microorganisms.
Artificial intelligence serves as the bridge between raw MEV data and actionable diagnostic tools. The development workflow follows a systematic process: data collection, preprocessing, model training, and validation. Large datasets of MEV profiles from both diseased and healthy individuals form the foundation, with standardization of collection and processing methods ensuring data quality.
Machine learning algorithms prove particularly effective in identifying diagnostic patterns within complex MEV datasets. Logistic Regression (LR) remains a staple for its interpretability, while more complex models like Gradient Boosting Machines (GBM) and Artificial Neural Networks (ANN) often deliver superior performance. Ensemble methods, which combine predictions from multiple models, frequently outperform individual algorithms. A notable example comes from lung cancer research, where an ensemble of GBM and ANN achieved an AUC of 0.94, surpassing the performance of either model alone.
Feature selection represents a critical step in model optimization. Techniques like LASSO regression, which outperforms traditional stepwise regression in many scenarios, help identify the most diagnostically relevant MEV markers. This process prioritizes markers based on their actual diagnostic utility rather than mere statistical significance, ensuring clinical relevance. The optimal combination of markers often exceeds the performance of individual biomarkers, highlighting the importance of combinatorial approaches.

MEV-based diagnostic models have demonstrated impressive performance across a spectrum of diseases, often outperforming conventional microbiome-based approaches. Colorectal cancer diagnostics using fecal MEVs showcase this potential, with one model incorporating Collinsella and Solanum melongena achieving an AUC of 0.95. Adding metabolomic markers (leucine and oxalic acid) further improved this to a perfect AUC of 1.00, illustrating the power of multi-omics integration.
Solid tumor diagnostics consistently show strong performance. Breast cancer models using blood MEVs achieved AUC values of 0.99 with LR and 1.00 with GBM when incorporating age as a covariate. Brain tumor diagnostics utilizing serum MEV profiles reached an AUC of 0.96 with LR and 0.99 with GBM, demonstrating the versatility of these approaches across different cancer types.
Infectious and inflammatory conditions also benefit from MEV-based diagnostics. Asthma diagnosis using IgG levels against specific MEVs achieved an AUC of 0.78, while COPD diagnostics reached 0.79 using similar approaches. These models often improve when incorporating disease-specific covariates, such as smoking status for respiratory conditions.
Sample type optimization significantly impacts performance. For gastric cancer, urine MEV-based models (AUC 0.82) outperformed those using fecal samples (AUC 0.64). This highlights the importance of selecting the most appropriate biological matrix for each disease application.
The utility of MEV-AI diagnostics extends beyond individual disease detection to comprehensive healthcare system integration. These tools enable precise risk stratification, identifying high-risk individuals before clinical symptoms appear. This proactive approach allows for targeted preventive interventions, potentially reducing disease incidence and severity in vulnerable populations.
In therapeutic settings, MEV-based models facilitate precision medicine by predicting treatment responses. This capability enables clinicians to select optimal therapeutic regimens based on individual MEV profiles, maximizing efficacy while minimizing adverse effects. Continuous monitoring using MEV biomarkers allows for real-time assessment of treatment response, enabling timely therapeutic adjustments.

MEV diagnostics also show promise in prognostic applications. Changes in MEV profiles following interventions like surgery or pharmaceutical therapy correlate with treatment outcomes, providing valuable prognostic information. This allows for more accurate patient counseling and better-informed treatment planning.
Despite significant progress, several challenges must be overcome for widespread clinical adoption. Method standardization represents a primary hurdle, with variations in ultracentrifugation protocols, NGS platforms, and bioinformatics pipelines leading to inconsistent results. Establishing universal quality control criteria for MEV purity and quantity will be essential for comparability between studies.
Sample size and diversity limitations currently restrict generalizability. Most studies involve small cohorts from single institutions or regions, failing to account for the substantial microbiome variability introduced by environmental factors like diet, geography, and lifestyle. International collaborative efforts to collect large, diverse datasets will be necessary to develop globally applicable diagnostic models.
Technological limitations include the predominantly genus-level resolution of current metagenomic analyses. While shotgun sequencing offers species-level identification, its higher cost and computational demands hinder widespread adoption. Advances in sequencing technology and bioinformatics efficiency will likely address this limitation in the coming years.
The future of MEV-AI diagnostics promises expanded applications and improved performance. Standardization initiatives will enhance reproducibility, while larger, more diverse datasets will increase generalizability. Advances in AI, particularly deep learning approaches, will enable more accurate predictions from increasingly complex multi-omics datasets.
Therapeutic applications of MEVs themselves represent another promising frontier. MEVs derived from beneficial microbes like Akkermansia muciniphila and Lactobacillus plantarum already show therapeutic potential in inflammatory and metabolic diseases. Their natural properties as nanocarriers also make them ideal for targeted drug delivery, combining diagnostic and therapeutic capabilities in a single platform.
The integration of MEV diagnostics into routine clinical practice will fundamentally transform healthcare delivery. By enabling earlier disease detection, more precise risk assessment, and personalized treatment strategies, these tools have the potential to significantly improve patient outcomes while reducing healthcare costs through targeted interventions. As technology advances and limitations are addressed, MEV-AI diagnostics stand poised to become cornerstones of 21st-century personalized medicine.
If you have related needs, please feel free to contact us for more information or product support.
Reference
This article is for research use only. Do not use in any diagnostic or therapeutic application.

Cat.No. GP-DQL-00203
Rotavirus Antigen Group A and Adenovirus Antigen Rapid Test Kit (Colloidal Gold)

Cat.No. GP-DQL-00206
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Card Style

Cat.No. GP-DQL-00207
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. GP-DQL-00211
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. GP-DQL-00212
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. IP-00189
Influenza A Rapid Assay Kit

Cat.No. GH-DQL-00200
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold)
Cat.No. GH-DQL-00201
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00202
Luteinizing Hormone Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00208
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold), Strip Style
Cat.No. GH-DQL-00209
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit(Colloidal Gold), Strip Style

Cat.No. GH-DQL-00210
Luteinizing Hormone Rapid Test Kit (Colloidal Gold), Strip Style
Cat.No. IH-HYW-0001
hCG Pregnancy Test Strip
Cat.No. IH-HYW-0002
hCG Pregnancy Test Cassette
Cat.No. IH-HYW-0003
hCG Pregnancy Test Midstream

Cat.No. GD-QCY-0001
Cocaine (COC) Rapid Test Kit

Cat.No. GD-QCY-0002
Marijuana (THC) Rapid Test Kit
Cat.No. GD-QCY-0003
Morphine (MOP300) Rapid Test Kit
Cat.No. GD-QCY-0004
Methamphetamine (MET) Rapid Test Kit
Cat.No. GD-QCY-0005
Methylenedioxymethamphetamine ecstasy (MDMA) Rapid Test Kit
Cat.No. GD-QCY-0006
Amphetamine (AMP) Rapid Test Kit
Cat.No. GD-QCY-0007
Barbiturates (BAR) Rapid Test Kit

Cat.No. GD-QCY-0008
Benzodiazepines (BZO) Rapid Test Kit
Cat.No. GD-QCY-0009
Methadone (MTD) Rapid Test Kit
Cat.No. GD-QCY-0011
Opiate (OPI) Rapid Test Kit
Cat.No. ID-HYW-0002
Multi-Drug Test L-Cup, (5-16 Para)
Cat.No. ID-HYW-0005
Multi-Drug Rapid Test (Dipcard & Cup) with Fentanyl
Cat.No. ID-HYW-0006
Multi-Drug Rapid Test (Dipcard & Cup) without Fentanyl
Cat.No. ID-HYW-0007
Multi-Drug 2~14 Drugs Rapid Test (Dipstick & Dipcard & Cup)
Cat.No. ID-HYW-0008
Fentanyl (FYL) Rapid Test (For Prescription Use)
Cat.No. ID-HYW-0009
Fentanyl Urine Test Cassette (CLIA Waived)
Cat.No. ID-HYW-0010
Fentanyl Urine Test Cassette (Home Use)
|
There is no product in your cart. |