- Home
- Resource
- Disease Diagnosis
- Metabolic Diseases
- Methylmalonic Acidemia (MMA): A Guide to Biomarker Profiling and Genetic Subtyping
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Methylmalonic Acidemia (MMA) is an inherited metabolic disorder caused by defects in methylmalonyl-CoA mutase or cobalamin metabolism, leading to toxic accumulation of methylmalonic acid and related metabolites. This resource systematically summarizes key diagnostic biomarkers, genotyping, differential diagnosis, and specialized in vitro diagnostic (IVD) products for MMA.
Methylmalonic acidemia (MMA) is an inherited metabolic disorder characterized by the body's inability to properly break down specific amino acids and lipids. This defect stems from a deficiency in methylmalonyl-CoA mutase (MCM) or its essential cofactor, adenosylcobalamin (a form of vitamin B12), leading to the toxic accumulation of methylmalonic acid and other compounds in the body. MMA typically presents in infancy with life-threatening symptoms like vomiting, lethargy, and metabolic acidosis. Precise diagnosis, through the identification of characteristic biomarkers and genetic subtyping, is critical for initiating targeted management, which may include dietary modification and vitamin B12 supplementation, to improve clinical outcomes.
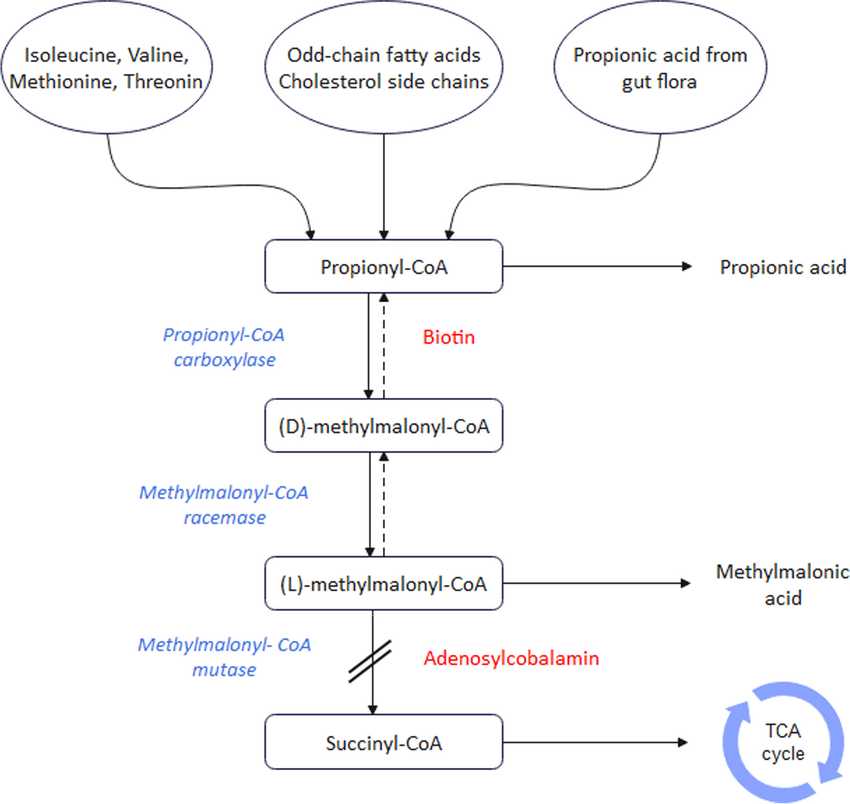 Fig.1 The metabolic pathway of methylmalonic acidemia (MMA). (Hakimzadeh Z, et al., 2024)
Fig.1 The metabolic pathway of methylmalonic acidemia (MMA). (Hakimzadeh Z, et al., 2024)
The diagnosis of methylmalonic acidemia (MMA) relies heavily on the detection of a characteristic profile of specific biomarkers across different bodily fluids. These biomarkers not only confirm the disease but also provide crucial clues for initial subtyping.
Propionylcarnitine (C3) is the primary screening biomarker for MMA. It accumulates in blood due to the blockage in propionate metabolism and is readily detected via tandem mass spectrometry in newborn screening programs. An elevated C3 level, especially when considered as a ratio to acetylcarnitine (C2), provides the initial, critical alert that prompts further diagnostic investigation.

Methylmalonic acid is the pathognomonic confirmatory biomarker for MMA. Its significant elevation in urine, identified through organic acid analysis, is a definitive diagnostic finding that confirms the organic acidemia. The direct accumulation of this compound in bodily fluids is responsible for much of the metabolic acidosis and toxicity seen in affected individuals.
Plasma total homocysteine (tHcy) is a crucial differentiating biomarker for subtyping MMA. It distinguishes between isolated MMA, where tHcy is typically normal or mildly elevated (e.g., MUT, cblA, cblB defects), and combined remethylation defects, where tHcy is significantly elevated (e.g., cblC defect). This measurement directly guides genetic testing and has profound implications for treatment, as B12-responsive forms often involve hyperhomocysteinemia.
Methylmalonic acidemia (MMA) genotyping is the process of identifying the specific genetic mutations responsible for an individual's disorder, moving beyond biochemical confirmation to define the precise molecular etiology. This is primarily achieved through DNA sequencing techniques, such as next-generation sequencing (NGS) panels, which analyze a curated set of genes known to cause MMA and related disorders. The primary goal is to identify biallelic pathogenic variants (mutations in both copies of a gene) that disrupt the function of the methylmalonyl-CoA mutase (MUT) enzyme or its essential cofactor, adenosylcobalamin (AdoCbl).
| Gene / Locus | Complementation Group | Key Biochemical Characteristics | Clinical & Therapeutic Implications |
| MUT | Mut | Isolated MMA. Severely elevated methylmalonic acid and C3. Normal plasma homocysteine. | Most severe form. Typically B12-nonresponsive. Prognosis correlates with residual enzyme activity (Mut⁰ > Mut⁻). |
| MMAA | cblA | Isolated MMA. Severely elevated methylmalonic acid and C3. Normal plasma homocysteine. | Often B12-responsive. Generally milder clinical presentation compared to MUT defects. |
| MMAB | cblB | Isolated MMA. Severely elevated methylmalonic acid and C3. Normal plasma homocysteine. | Variable B12-responsiveness. Clinical severity is often intermediate. |
| MMACHC | cblC | Combined MMA with Homocystinuria. Elevated methylmalonic acid, C3, and markedly elevated plasma homocysteine. | The most common cobalamin disorder. Treatment involves hydroxocobalamin, betaine, and other supplements. Phenotype can be severe. |
| MMADHC | cblD | Variable. cblD variant 1: presents like cblC (MMA + homocystinuria). cblD variant 2: presents like cblA/cblB (isolated MMA). | Management depends on the specific biochemical phenotype (i.e., whether homocystinuria is present). |
| HCFC1 | cblX | Combined MMA with Homocystinuria. Similar biochemical profile to cblC. | Often associated with significant neurological features and intellectual disability. |
The differential diagnosis for methylmalonic acidemia (MMA) primarily involves distinguishing it from other disorders that present with similar clinical features or overlapping biomarker patterns, particularly an elevated propionylcarnitine (C3). Key conditions to rule out include propionic acidemia (PA), which shares an identical newborn screening result and acute clinical presentation, and disorders of cobalamin (B12) metabolism that cause isolated homocystinuria without MMA. Additionally, nutritional B12 deficiency in the mother can cause elevated C3 and methylmalonic acid in the infant. Definitive differentiation relies on a specific biochemical profile—notably, the pathognomonic elevation of methylmalonic acid and methylcitrate on urine organic acid analysis for MMA—and is confirmed through targeted genetic testing to identify the specific enzymatic defect.
Alta DiagnoTech delivers end-to-end IVD solutions for methylmalonic acidemia (MMA), offering a complete portfolio of diagnostic products for clinical testing and specialized research. Our integrated approach supports the entire workflow from initial screening to genetic confirmation, providing healthcare professionals and researchers with the precise tools needed to advance the diagnosis and understanding of MMA. If you have related needs, please feel free to contact us for more information or product support.
| Newborn Screening Products |
|
| Biochemical Confirmation & Monitoring Kits |
|
| Genetic Subtyping & Confirmation |
|
| Specialty & Research-Use Only (RUO) Products |
|
References
This article is for research use only. Do not use in any diagnostic or therapeutic application.
|
There is no product in your cart. |