- Home
- Resource
- Explore & Learn
- Market Trends and Future Outlook for Cancer Molecular Diagnostic Kits
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Molecular diagnostics represents a paradigm shift in cancer diagnosis, leveraging advancements in genomics, proteomics, and bioinformatics to provide precise, early, and actionable insights into cancer biology. Unlike traditional methods that rely on morphological changes in cells, molecular diagnostics focus on detecting specific genetic alterations, mutations, and biomarkers associated with cancer initiation, progression, and response to therapy.
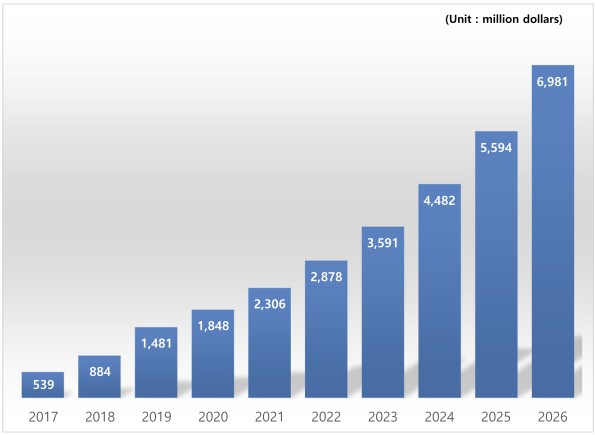 Fig.1 Forecasts for the global market for blood cancer molecular diagnostics kits. (Seo J. H., et al., 2018)
Fig.1 Forecasts for the global market for blood cancer molecular diagnostics kits. (Seo J. H., et al., 2018)

PCR is a cornerstone technology in molecular diagnostics, allowing for the amplification of specific DNA sequences from minute quantities of starting material. Real-time PCR (rt-PCR) and digital PCR (d-PCR) variants offer exceptional sensitivity and quantitative capabilities, making them ideal for detecting low-abundance cancer biomarkers. PCR-based kits are widely used in diagnosing blood cancers, such as leukemia and lymphoma, where chromosomal translocations and gene mutations are common.

NGS technologies have revolutionized cancer genomics by enabling the simultaneous sequencing of millions of DNA fragments. This high-throughput approach allows for comprehensive profiling of tumor genomes, identifying somatic mutations, copy number alterations, and structural variants that drive cancer development. NGS-based molecular diagnostics kits are increasingly being used to guide targeted therapies and monitor treatment response in real-time.
Microarrays and FISH are powerful tools for detecting genomic alterations, such as copy number variations and gene fusions. Microarrays consist of thousands of DNA probes immobilized on a solid surface, allowing for the simultaneous analysis of multiple genetic loci. FISH, on the other hand, uses fluorescently labeled DNA probes to visualize specific chromosomal regions or gene sequences within cells. Both technologies are valuable in diagnosing hematological malignancies and solid tumors with known genetic abnormalities.

Global Market Dynamics
The global market for cancer molecular diagnostics kits is experiencing robust growth, driven by technological advancements, increasing cancer incidence, and a shift towards preventive healthcare and personalized medicine. According to recent market analyses, the global market for blood cancer molecular diagnostics kits alone was valued at 335.9millionin2016andisprojectedtoreach6.98 billion by 2026, growing at an average annual rate of 32.9%.

Regional Market Insights
North America and Europe dominate the global market for cancer molecular diagnostics kits, owing to well-established healthcare infrastructures, high awareness levels, and favorable reimbursement policies. However, the Asia-Pacific region is emerging as a significant growth market, driven by rising healthcare expenditures, improving access to medical technologies, and a large patient population. South Korea, in particular, is anticipated to experience substantial growth in the blood cancer molecular diagnostics market, with projections indicating an increase from 3.75millionin2016to60.89 million by 2026.
Competitive Landscape
The molecular diagnostics market is characterized by a high degree of concentration, with a few global players accounting for a significant share of the market. Companies such as Roche, Qiagen, and Abbott Laboratories lead the market, leveraging their extensive product portfolios, strong research and development capabilities, and global distribution networks. However, the market also presents opportunities for smaller, innovative firms to carve out niches by focusing on specific cancer types, technologies, or geographic regions.
If you have related needs, please feel free to contact us for more information or product support.
Reference
We provide molecular diagnostic kits, which are efficient tools designed for molecular biology research and clinical diagnosis and can accurately detect changes in nucleic acid (DNA/RNA) sequences, structures, or expression levels in biological samples, which help medical research such as disease diagnosis, pathogen detection, and genetic disease screening. Specifically covering the following types:

This article is for research use only. Do not use in any diagnostic or therapeutic application.
|
There is no product in your cart. |