- Home
- Resource
- Disease Diagnosis
- Cancers
- Liver Cancer Blood-Based Diagnostics: A Guide to Key Biomarkers and Assays
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Liver cancer, primarily hepatocellular carcinoma (HCC), is a major global health challenge whose prognosis heavily depends on early and accurate detection. This resource page provides a comprehensive guide to the blood-based diagnostic landscape, detailing the established and emerging biomarkers, from serum proteins like AFP and PIVKA-II to circulating tumor DNA (ctDNA), and explaining the core IVD technologies that power their analysis.
Liver cancer, predominantly hepatocellular carcinoma (HCC), is a major global health challenge and a leading cause of cancer-related mortality. It typically arises on a background of chronic liver disease, with key risk factors including chronic hepatitis B (HBV) or C (HCV) infection, alcohol-related liver disease, and metabolic dysfunction-associated steatotic liver disease (MASLD). The insidious nature of the disease, often remaining asymptomatic until advanced stages, contributes to its high mortality rate. This underscores the critical importance of effective surveillance and early detection strategies in at-risk populations to significantly improve patient outcomes.
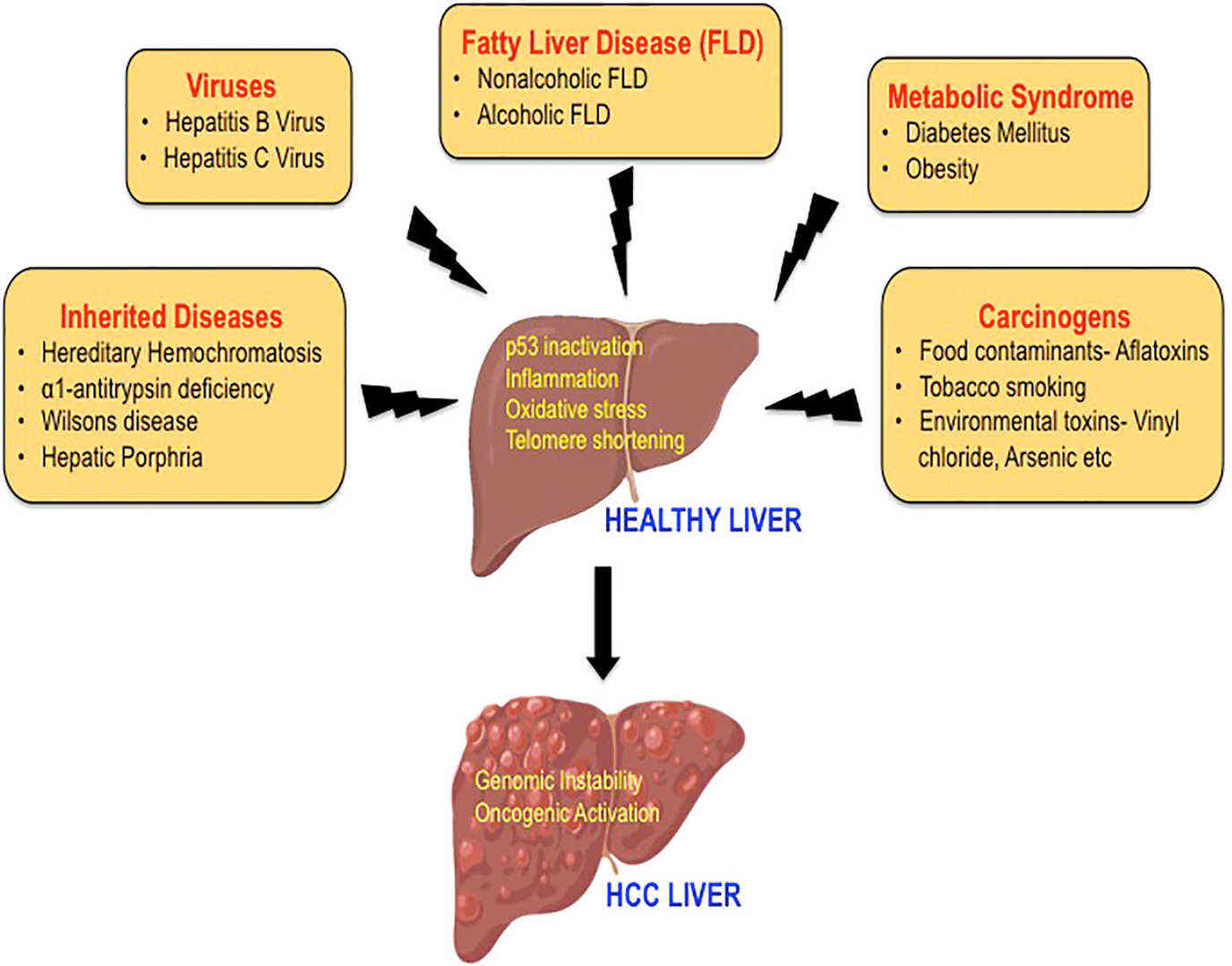 Fig.1 The etiology of hepatocellular carcinoma. (Suresh D, et al., 2020)
Fig.1 The etiology of hepatocellular carcinoma. (Suresh D, et al., 2020)
Blood-based diagnostics are revolutionizing the management of liver cancer by offering a minimally invasive approach for detection, monitoring, and prognostication. These liquid biopsy techniques analyze circulating biomarkers in the blood, including traditional serum proteins like Alpha-fetoprotein (AFP) and its specific isoform AFP-L3, as well as Des-gamma-carboxy Prothrombin (DCP). Increasingly, they also encompass advanced molecular markers such as circulating tumor DNA (ctDNA) and circulating tumor cells (CTCs). By providing a real-time snapshot of the tumor's activity, these tests are invaluable for the early detection of hepatocellular carcinoma (HCC) in high-risk patients, assessing treatment response, and monitoring for disease recurrence, thereby enabling more personalized and proactive patient care.
The accurate diagnosis and management of liver cancer, particularly hepatocellular carcinoma (HCC), are increasingly guided by the detection of specific biomarkers in the blood. These biomarkers, which can be proteins, genetic materials, or entire cells shed by tumors, provide critical, minimally invasive insights for early detection, risk stratification, and treatment monitoring.
Established Serum Protein Biomarkers
Emerging Biomarkers
The accurate detection and quantification of liver cancer biomarkers rely on a suite of sophisticated core in vitro diagnostic (IVD) technologies. These assays are the essential tools that translate a blood sample into a reliable, clinically actionable result, enabling everything from early detection to treatment monitoring.

Immunoassays are the foundational technology for measuring protein biomarkers, such as AFP, AFP-L3, and DCP. These tests use highly specific antibodies that bind to the target protein, generating a detectable signal (e.g., light or color) that is proportional to the concentration of the biomarker in the sample. Common platforms include ELISA and more advanced, automated CLIA and ECLIA, which offer high sensitivity and throughput for clinical laboratories.

Molecular diagnostics are used to detect and analyze nucleic acid-based biomarkers, primarily circulating tumor DNA (ctDNA). These techniques identify specific genetic mutations (e.g., in the TERT promoter), methylation patterns, or other genomic alterations. Key technologies include PCR-based methods like digital PCR (dPCR) for highly sensitive detection of known mutations, and NGS, which provides a comprehensive, unbiased profile of multiple genetic alterations from a single liquid biopsy sample.

This is a specialized hybrid technique used specifically to separate and measure the AFP-L3 isoform. It leverages the unique binding property of a lectin (Lens culinaris agglutinin) to the fucose sugar residue on AFP-L3. The sample is passed through a lectin-bound cartridge, which captures the AFP-L3 fraction. The captured fraction is then typically quantified using a standard immunoassay, providing the critical %AFP-L3 ratio that is indicative of aggressive HCC.
At Alta DiagnoTech, we are committed to advancing the fight against liver cancer by providing a comprehensive portfolio of high-performance in vitro diagnostic (IVD) solutions. Our products span the entire clinical journey, from early risk assessment and accurate diagnosis to proactive treatment monitoring, empowering clinicians with reliable, actionable data to improve patient outcomes. If you have related needs, please feel free to contact us for more information or product support.
| Product Name | Technology | Application |
| AFP Chemiluminescent Immunoassay Kit | Chemiluminescence | Quantitative serum AFP for HCC surveillance |
| AFP-L3 Auto-assay | Microfluidic CLIA | %AFP-L3 ratio for early-HCC risk assessment |
| PIVKA-II (DCP) Immunoassay Kit | Electrochemiluminescence | Serum DCP quantitation, HCC detection |
| AFP-L3 + PIVKA-II Combo Panel | Multiplex CLIA | Simultaneous AFP-L3 & DCP for improved early-HCC pick-up |
| HB miRDx EarlyHCC Kit | RT-qPCR (plasma) | Circulating miRNA panel detecting Stage 1-4 HCC |
| μTASWako i30 AFP-L3 Test | Micro-chip affinity electrophoresis | %AFP-L3 on routine chemistry analyzer |
| μTASWako i30 DCP Test | Micro-chip immunoassay | Serum DCP for HCC surveillance |
| AFP ELISA Kit | ELISA | Research/clinical quantitation of total AFP |
Reference
This article is for research use only. Do not use in any diagnostic or therapeutic application.
|
There is no product in your cart. |