Lateral flow assays (LFAs) have emerged as indispensable tools in the field of clinical diagnostics, particularly for point-of-care testing (POCT). These assays are renowned for their simplicity, rapid results, and cost-effectiveness, making them ideal for detecting biomarkers in various biological samples such as blood, urine, and saliva. However, traditional LFAs often face significant challenges in sensitivity and specificity, primarily due to background autofluorescence from biological samples or the detection matrix. This interference can lead to false negatives or reduced accuracy, particularly when detecting low-abundance biomarkers. To address these limitations, researchers have turned to innovative solutions involving low-background luminescent nanomaterials (LBLNPs), which offer enhanced sensitivity and reliability.
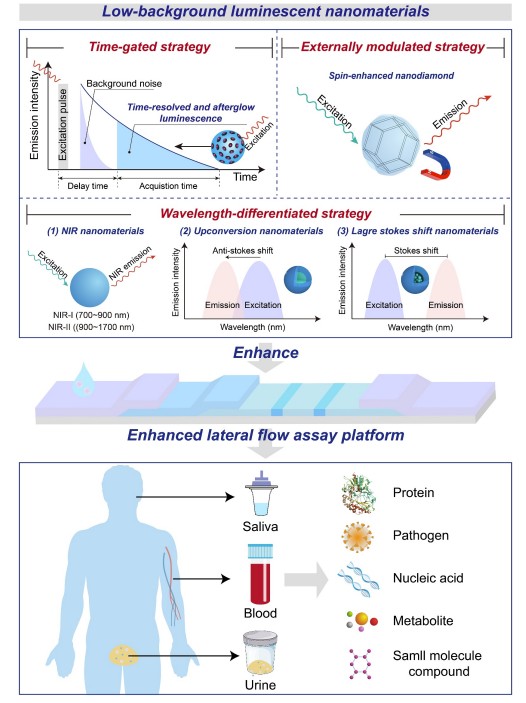 Fig.1 Schematic illustration of various low-background luminescent nanoparticles-enhanced lateral flow assay platforms for clinical diagnosis. (Hao L., et al., 2025)
Fig.1 Schematic illustration of various low-background luminescent nanoparticles-enhanced lateral flow assay platforms for clinical diagnosis. (Hao L., et al., 2025)
Time-Gated Luminescence: A Breakthrough in Sensitivity
Time-gated luminescence is a technique that leverages the temporal properties of certain nanomaterials to separate the detection signal from background noise. This approach includes time-resolved fluorescence (TRF) and afterglow luminescence, both of which utilize the delayed emission of light to enhance the signal-to-noise ratio (SNR).
Time-Resolved Fluorescence Nanomaterials (TRFNs)
TRFNs are engineered to emit fluorescence with a delayed emission time, typically ranging from microseconds to milliseconds. This delay allows for the detection of the TRFN signal after the short-lived background autofluorescence has decayed. TRFNs are often based on lanthanide elements such as europium (Eu), terbium (Tb), dysprosium (Dy), and gadolinium (Ga), which are known for their long-lived luminescence properties. For instance, Hu et al. demonstrated that TRFN-based LFAs for detecting ractopamine in swine urine were significantly more sensitive than LFAs using fluorescent submicrospheres, quantum dots, and colloidal gold. This enhanced sensitivity is attributed to the minimal background signal conferred by the prolonged luminescence of TRFNs.
Afterglow Luminescence Nanomaterials (ALNPs)
Afterglow luminescence nanomaterials take the concept of delayed emission even further, with luminescent signals that can last for seconds to days after excitation. This long-lived emission makes ALNPs particularly useful in environments with high autofluorescence, as they can be detected long after the excitation source has been removed. ALNPs can be categorized into inorganic and organic materials. Inorganic ALNPs, such as zinc gallate doped with chromium (Cr3+) or strontium aluminate doped with europium (Eu2+) and dysprosium (Dy3+), are known for their robustness and long-lived luminescence. For example, Paterson et al. used europium- and dysprosium-doped strontium aluminate phosphors to develop an LFA with a limit of detection (LOD) of 100 pg/mL for biotinylated hen egg lysozyme, which is an order of magnitude more sensitive than conventional gold nanoparticles.
Wavelength-Differentiated LBLNPs: Enhancing Detection Through Spectral Separation
Another strategy to minimize background interference is to use nanomaterials that emit light in regions of the spectrum where autofluorescence is minimal. This includes near-infrared (NIR) luminescence, upconversion luminescence, and large Stokes shift fluorescence.
Near-Infrared Luminescence Nanomaterials (NIR NPs)
NIR luminescence nanomaterials operate in the 700-1700 nm wavelength range, where light absorption and scattering by biological tissues are minimal. This results in higher tissue penetration and reduced background noise, making NIR NPs ideal for detecting low-abundance biomarkers. For example, Fan et al. developed an NIR-I fluorescent nanoprobe (800CW) for detecting 5-hydroxyflunixin in raw milk, achieving a LOD of 0.073 ng/mL, which is 11.2 times more sensitive than gold nanoparticle-based LFAs. Similarly, Song et al. used NIR-II luminescent nanoparticles to detect carcinoembryonic antigen (CEA) in serum with a LOD of 0.37 ng/mL, which is 13.5 times higher than the clinical cutoff value.
Upconversion Luminescence Nanomaterials (UCNPs)
UCNPs convert low-energy light into higher-energy light, a phenomenon known as anti-Stokes shift. This unique property allows UCNPs to emit light in the NIR range, reducing background interference from biological tissues. For instance, Ji et al. developed a NIR-to-NIR UCNPs-enhanced LFA for detecting procalcitonin (PCT) in human plasma with a LOD of 0.03 ng/mL, which is lower than normal human plasma levels.
Large Stokes Shift Fluorescent Nanomaterials
Large Stokes shift fluorescent nanomaterials have a significant difference between their excitation and emission wavelengths, reducing overlap and background fluorescence. For example, Seo et al. encapsulated fluorophores with large Stokes shifts into polystyrene nanoparticles to enhance LFA sensitivity, achieving a LOD of 1 ng/mL for SARS-CoV-2 spike protein, which is 100 times more sensitive than gold nanoparticle-based LFAs.
External Modulated LBLNPs: Spin-Enhanced Fluorescent Nanodiamonds
Spin-enhanced fluorescent nanodiamonds (FNDs) containing nitrogen-vacancy (NV) centers offer a unique approach to reducing background noise. These nanodiamonds exhibit spin-dependent fluorescence that can be modulated by external magnetic or microwave fields. This allows for selective detection of the NV center fluorescence, effectively filtering out background autofluorescence. For example, Miller et al. used FNDs to enhance LFA sensitivity, achieving a 105-fold increase in sensitivity compared to gold nanoparticle-based LFAs. Le et al. developed a spin-enhanced LFA for detecting dengue virus with a LOD of 0.1 ng/mL, demonstrating the potential of FNDs for ultrasensitive detection.
Future Directions and Challenges
While the development of LBLNPs has significantly advanced the field of lateral flow assays, several challenges remain. These include the need for large-scale synthesis techniques that maintain reproducibility, the integration of LBLNPs with high-affinity biorecognition agents, and the development of affordable, portable readout instruments for quantitative analysis.
- Large-Scale Synthesis and Reproducibility
One of the primary challenges in the widespread adoption of LBLNPs is the scalability of their synthesis. Current methods often yield low reproducibility, which can compromise the accuracy and reliability of LFAs. Microfluidic platform-based synthesis strategies offer a promising solution, providing controlled and consistent production of nanomaterials.
- Integration with High-Affinity Biorecognition Agents
The functionalized surface of nanomaterials determines the detection stability and anti-interference ability of LFAs platforms when faced with complex sample matrices. Effective surface coating agents and modifiers are essential for preparing LBLNPs that can better fulfill the requirements of precise clinical diagnostics. Additionally, the integration of LBLNPs with high-affinity biorecognition agents such as antibodies or aptamers is crucial for enhancing specific recognition and reducing non-specific binding events.
- Development of Portable Readout Instruments
The signal readout modes for LBLNPs-based LFAs often require specialized instruments, which can compromise user-friendliness and escalate detection costs. There is a pressing need for the development of affordable, compact, portable, and user-friendly readout instruments for the quantitative analysis of low-background LFAs. These instruments should be capable of providing rapid, accurate, and quantitative results without requiring extensive technical expertise.
Conclusion
The advent of low-background luminescent nanomaterials represents a significant leap forward in the sensitivity and reliability of lateral flow assays. By minimizing background interference and enhancing signal detection, these nanomaterials offer the potential to revolutionize point-of-care testing, enabling earlier and more accurate diagnosis of diseases. As research continues to advance, we can expect to see these innovative materials become an integral part of clinical diagnostic tools, ultimately improving patient outcomes and healthcare delivery. The future of clinical diagnosis lies in the continued development and refinement of these cutting-edge technologies, ensuring that they are accessible, affordable, and effective for widespread use.
If you have related needs, please feel free to contact us for more information or product support.
Reference
- Hao, Liangwen, et al. "Recent advances in low-background luminescent nanomaterials-enhanced lateral flow assays for clinical diagnosis." Interdisciplinary Medicine (2025): e20240078.
This article is for research use only. Do not use in any diagnostic or therapeutic application.
Trending Products



 Fig.1 Schematic illustration of various low-background luminescent nanoparticles-enhanced lateral flow assay platforms for clinical diagnosis. (Hao L., et al., 2025)
Fig.1 Schematic illustration of various low-background luminescent nanoparticles-enhanced lateral flow assay platforms for clinical diagnosis. (Hao L., et al., 2025)