- Home
- Resource
- Explore & Learn
- Innovation and Hurdles in Molecular Point-of-Care Testing Platforms for Pathogens Utilizing Microfluidic Technology
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Microfluidic molecular point-of-care testing (POCT) systems represent a transformative advancement in the field of in vitro diagnostics, particularly for infectious diseases. These systems integrate the complexities of traditional laboratory processes into a compact, portable, and automated platform, significantly reducing the time and resources required for diagnosis. By leveraging microfluidic technology, these devices can perform nucleic acid detection, amplification, and analysis directly at the point of care, providing rapid and accurate results that can guide immediate clinical decision-making.
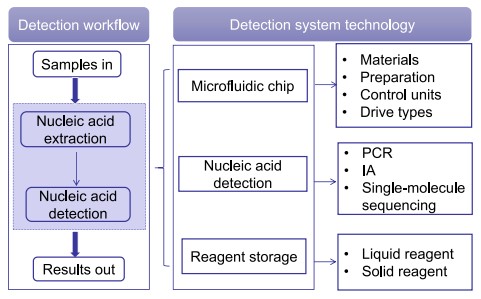 Fig.1 Modules of molecular POCT systems. (Ren S., et al., 2025)
Fig.1 Modules of molecular POCT systems. (Ren S., et al., 2025)
Microfluidic chips are the cornerstone of molecular POCT systems, integrating sample processing, nucleic acid extraction, amplification, and detection into a single device. These chips are fabricated from materials such as silicon, glass, polydimethylsiloxane (PDMS), and polymethyl methacrylate (PMMA), each offering unique advantages in terms of biocompatibility, transparency, and ease of fabrication. For instance, PDMS is favored for its flexibility and low cost, while glass provides superior optical properties for detection.
The fabrication process involves techniques like photolithography, soft lithography, and injection molding, allowing for the creation of intricate microchannels and reaction chambers. Surface modification techniques further enhance chip performance by optimizing hydrophilicity and reducing nonspecific binding. These advancements enable the precise manipulation of minute fluid volumes, facilitating highly sensitive and specific diagnostic assays.

Variable-Temperature Amplification: PCR
Polymerase chain reaction (PCR) remains a gold standard for nucleic acid amplification due to its high sensitivity and specificity. Traditional PCR requires thermal cycling to denature, anneal, and extend nucleic acid sequences. However, integrating PCR into microfluidic chips presents challenges related to rapid and uniform heating and cooling. Recent innovations in microfluidic PCR systems have addressed these issues by optimizing chip design to enhance heat transfer, thereby reducing thermal cycling times and improving reaction efficiency.
Isothermal Amplification: A Game Changer
Isothermal amplification techniques, such as loop-mediated isothermal amplification (LAMP) and recombinase polymerase amplification (RPA), have revolutionized point-of-care diagnostics by eliminating the need for thermal cycling. These methods operate at a constant temperature, simplifying the device requirements and making them ideal for portable applications. LAMP, for example, amplifies nucleic acids rapidly and can be visually detected using colorimetric indicators, making it user-friendly for non-laboratory settings.
Single-Molecule Sequencing: The Frontier of Real-Time Detection
Single-molecule sequencing technologies, including single-molecule real-time (SMRT) sequencing and nanopore sequencing, offer unprecedented capabilities for real-time nucleic acid detection. These methods bypass the need for amplification, reducing the risk of contamination and enabling direct sequencing of pathogens. Nanopore sequencing, in particular, has shown promise for rapid detection of emerging pathogens, such as Ebola and SARS-CoV-2, in resource-limited settings due to its portability and ease of use.

Effective reagent storage and release are critical for the functionality and shelf life of molecular POCT systems. Traditional methods involving external reagent injection are prone to contamination and operational errors. Modern microfluidic chips incorporate innovative storage solutions, such as solid reagents fixed on microbeads or within hydrogel matrices. These reagents can be rapidly reconstituted upon contact with samples, ensuring consistent and reliable performance.
Liquid reagents can be stored in sealed compartments within the chip, released through mechanisms like melting paraffin or puncturing foil sacs. These advancements enable the development of fully integrated, self-contained diagnostic devices that require minimal user intervention, enhancing their suitability for point-of-care applications.
Microfluidic molecular POCT systems have broad applications across various healthcare settings, from hospital emergency departments to remote clinics. Their portability and rapid results make them invaluable for diagnosing infectious diseases like influenza, tuberculosis, and COVID-19. For instance, the GeneXpert system, which utilizes microfluidic PCR technology, has been widely adopted for the rapid detection of Mycobacterium tuberculosis, significantly improving patient outcomes and reducing transmission rates.
In the context of global health emergencies, such as the COVID-19 pandemic, molecular POCT systems have played a crucial role in rapid testing and surveillance. These devices enable large-scale screening in community settings, facilitating the timely isolation and treatment of infected individuals. Additionally, their use in resource-limited regions helps bridge the diagnostic gap, ensuring equitable access to accurate testing.
Multi-Channel and High-Throughput Devices
The next generation of microfluidic molecular POCT systems is expected to feature multi-channel designs capable of processing multiple samples simultaneously. This advancement will enhance throughput and efficiency, making these devices even more suitable for high-demand settings like emergency departments and outbreak response centers. Integration with advanced detection technologies, such as electrochemical sensors and fluorescence microscopy, will further improve sensitivity and specificity.
Integration with Digital Health Platforms
The convergence of microfluidic technology with digital health platforms represents a significant frontier in diagnostic innovation. Connecting POCT devices to cloud-based systems enables real-time data sharing, facilitating remote monitoring and telemedicine applications. This integration can also support the development of personalized treatment plans based on individual patient data, enhancing the overall quality of care.
Despite the rapid advancements in microfluidic molecular POCT systems, challenges remain in standardizing these technologies. The complexity of microfluidic chip design and fabrication, combined with the diversity of materials and reagents used, complicates the establishment of unified quality control standards. Regulatory bodies, such as the FDA and EMA, are actively working to develop guidelines that ensure the safety, accuracy, and reliability of these devices.
Regulatory approval for molecular POCT systems involves rigorous validation of performance characteristics, including sensitivity, specificity, and reproducibility. Manufacturers must demonstrate that their devices meet stringent standards for clinical use. This process requires extensive collaboration between developers, regulatory agencies, and healthcare providers to ensure that these innovative technologies can be safely and effectively deployed in various settings.
Microfluidic molecular POCT systems are poised to redefine infectious disease diagnosis by offering rapid, accurate, and portable diagnostic solutions. Their integration of advanced nucleic acid detection technologies, innovative reagent storage mechanisms, and potential for digital connectivity underscores their transformative potential in healthcare. As these systems continue to evolve, addressing regulatory challenges and establishing standardized protocols will be crucial for their widespread adoption. The future of infectious disease diagnosis lies in the seamless integration of these cutting-edge technologies into everyday clinical practice, ultimately improving patient outcomes and public health.
If you have related needs, please feel free to contact us for more information or product support.
Reference
This article is for research use only. Do not use in any diagnostic or therapeutic application.

Cat.No. GP-DQL-00203
Rotavirus Antigen Group A and Adenovirus Antigen Rapid Test Kit (Colloidal Gold)

Cat.No. GP-DQL-00206
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Card Style

Cat.No. GP-DQL-00207
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. GP-DQL-00211
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. GP-DQL-00212
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. IP-00189
Influenza A Rapid Assay Kit

Cat.No. GH-DQL-00200
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00201
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00202
Luteinizing Hormone Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00208
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. GH-DQL-00209
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit(Colloidal Gold), Strip Style

Cat.No. GH-DQL-00210
Luteinizing Hormone Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. IH-HYW-0001
hCG Pregnancy Test Strip

Cat.No. IH-HYW-0002
hCG Pregnancy Test Cassette

Cat.No. IH-HYW-0003
hCG Pregnancy Test Midstream

Cat.No. GD-QCY-0001
Cocaine (COC) Rapid Test Kit

Cat.No. GD-QCY-0002
Marijuana (THC) Rapid Test Kit

Cat.No. GD-QCY-0003
Morphine (MOP300) Rapid Test Kit

Cat.No. GD-QCY-0004
Methamphetamine (MET) Rapid Test Kit

Cat.No. GD-QCY-0005
Methylenedioxymethamphetamine ecstasy (MDMA) Rapid Test Kit

Cat.No. GD-QCY-0006
Amphetamine (AMP) Rapid Test Kit

Cat.No. GD-QCY-0007
Barbiturates (BAR) Rapid Test Kit

Cat.No. GD-QCY-0008
Benzodiazepines (BZO) Rapid Test Kit

Cat.No. GD-QCY-0009
Methadone (MTD) Rapid Test Kit

Cat.No. GD-QCY-0011
Opiate (OPI) Rapid Test Kit

Cat.No. ID-HYW-0002
Multi-Drug Test L-Cup, (5-16 Para)

Cat.No. ID-HYW-0005
Multi-Drug Rapid Test (Dipcard & Cup) with Fentanyl

Cat.No. ID-HYW-0006
Multi-Drug Rapid Test (Dipcard & Cup) without Fentanyl

Cat.No. ID-HYW-0007
Multi-Drug 2~14 Drugs Rapid Test (Dipstick & Dipcard & Cup)

Cat.No. ID-HYW-0008
Fentanyl (FYL) Rapid Test (For Prescription Use)

Cat.No. ID-HYW-0009
Fentanyl Urine Test Cassette (CLIA Waived)

Cat.No. ID-HYW-0010
Fentanyl Urine Test Cassette (Home Use)
|
There is no product in your cart. |