- Home
- Resource
- Disease Diagnosis
- Infectious Diseases
- Human Papillomavirus (HPV) Diagnostics: From Screening to Genotyping
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Human papillomavirus (HPV) infection affects over 80% of sexually active people and is the leading cause of cervical cancer. It may also lead to other anogenital and oropharyngeal malignancies. This resource provides a comprehensive overview of HPV diagnostic biomarkers and modern diagnostic solutions, covering basic screening methods to advanced genotyping technologies.
Human papillomavirus (HPV) is the most common sexually transmitted infection globally, with over 200 genotypes classified as either low-risk (e.g., HPV-6/11, causing genital warts) or high-risk (e.g., HPV-16/18, responsible for 70% of cervical cancers and linked to other anogenital/oropharyngeal malignancies). Most infections are transient, but persistent high-risk HPV can lead to precancerous lesions, underscoring the critical need for accurate screening, genotyping, and triage strategies.
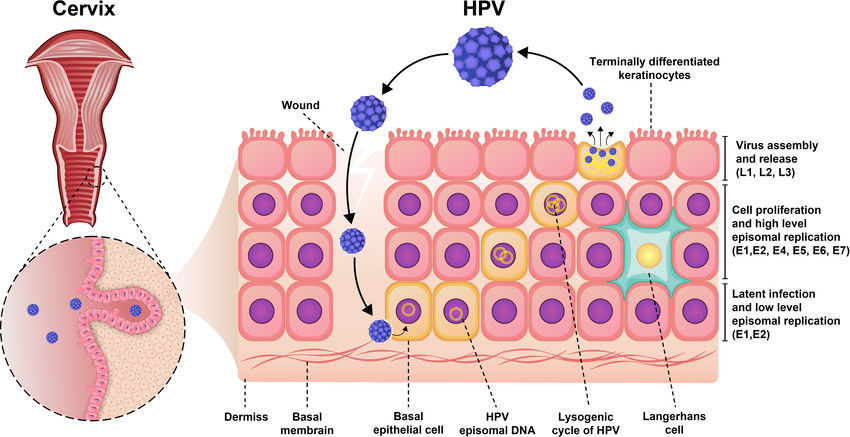 Fig.1 Pathogenesis of Human papillomavirus (HPV) infection. (Yousefi Z, et al., 2022)
Fig.1 Pathogenesis of Human papillomavirus (HPV) infection. (Yousefi Z, et al., 2022)
Human papillomavirus (HPV) is a small, non-enveloped DNA virus with two key protein groups that drive infection and oncogenesis: structural capsid proteins (L1/L2), which form the viral shell and are targeted by vaccines, and early regulatory proteins (E6/E7), which promote carcinogenesis by degrading tumor suppressors (p53 and pRb). These proteins serve as critical biomarkers.
| Biomarkers | Detection Method | Clinical Utility |
| HPV DNA | PCR or hybrid-capture assay on cervical swab/tissue | Primary screening for high-risk HPV types; risk stratification in cervical-cancer prevention programs |
| Viral Capsid Protein L1 | Immunocytochemistry (ICC) on cervical cytology slides | Adjunct marker to confirm productive HPV infection; loss of L1 expression suggests higher risk of progression |
| p16INK4a | Immunohistochemistry (IHC) or dual-stain ICC on cytology/biopsy | Triage of HPV-positive women; strong diffuse p16 staining indicates transforming infection (≥CIN2) |
| Ki-67 | Immunohistochemistry (IHC) or dual-stain ICC on cytology/biopsy | Combined with p16 to improve specificity; high Ki-67 index confirms active cellular proliferation in dysplastic lesions |
| E6/E7 Oncoproteins | Immunohistochemistry (IHC) for tissue sections; ELISA or multiplex for serum | Direct evidence of oncogene expression; serum assays useful for oropharyngeal cancer screening; tissue assays confirm HPV-driven malignancy |
Human papillomavirus (HPV) screening is critical for early detection of high-risk HPV (hrHPV) infections, which are responsible for nearly all cervical cancers and linked to other anogenital and oropharyngeal malignancies. Unlike cytology-based methods (e.g., Pap smears), primary HPV testing offers higher sensitivity (>90%) for identifying precancerous lesions, enabling timely intervention to prevent cancer progression.
Molecular HPV Testing
Molecular HPV tests detect high-risk HPV (hrHPV) DNA or RNA (e.g., E6/E7 mRNA) with high sensitivity (>90%) and are now the gold standard for primary screening. PCR-based assays identify 14 hrHPV types with genotyping for HPV-16/18, while RNA tests offer improved specificity by targeting oncogenic activity. These methods enable early detection of precancerous lesions and guide clinical management.
Cytology (Pap Smear)
The Pap smear microscopically examines cervical cells for dysplasia, traditionally serving as the primary screening tool. Though specific (~90%), its lower sensitivity (50–70%) compared to HPV testing has relegated it to a triage role for HPV+ cases or co-testing in some regions. Automation and AI-assisted analysis aim to reduce subjectivity in interpretation.
Emerging Methods
Innovations include self-sampling kits (validated with PCR assays) designed to improve accessibility and the ability to perform immediate isothermal amplification (e.g., LAMP) in resource-poor settings. Biomarkers such as p16/Ki-67 dual staining can improve triage accuracy. These advances aim to bridge the global screening gap.
Human papillomavirus (HPV) genotyping identifies specific high-risk (hrHPV) and low-risk (lrHPV) types, enabling precise risk stratification and clinical management. While screening tests often detect pooled hrHPV, genotyping distinguishes HPV-16/18 (responsible for ~70% of cervical cancers) from other hrHPV types (e.g., 31, 33, 45) or lrHPV (e.g., 6/11 causing warts).
PCR-based genotyping detects and differentiates HPV types by amplifying viral DNA using type-specific primers or broad-spectrum assays. These tests offer high throughput, clinical-grade accuracy, and are widely used for primary screening and triage, particularly to identify HPV-16/18.
Sanger or next-generation sequencing (NGS) provides comprehensive genotyping, identifying rare or novel HPV types beyond standard panels. While highly specific, sequencing is primarily used for research, epidemiology, or resolving equivocal PCR results, as its cost and complexity limit routine clinical use.
RNA assays target E6/E7 oncogenic mRNA, offering higher specificity for precancer risk than DNA tests. By focusing on actively transcribed viral oncogenes, these tests reduce false positives and better predict persistent infections, guiding management for HPV-16/18/45+ cases.
Alta DiagnoTech provides a one-stop solution for human papillomavirus (HPV) detection, encompassing high-precision PCR genotyping assay kits, rapid point-of-care test kits, and advanced sequencing-based typing systems to meet diverse clinical and research needs. If you have related needs, please feel free to contact us for more information or product support.
Reference
This article is for research use only. Do not use in any diagnostic or therapeutic application.
|
There is no product in your cart. |