- Home
- Resource
- Disease Diagnosis
- Infectious Diseases
- Herpes Simplex Virus (HSV) Diagnostics: From Lab-Based Tests to Point-of-Care Innovations
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Herpes simplex virus (HSV) infection requires accurate and timely diagnosis for effective clinical management and infection control. This resource provides a systematic review of current and emerging HSV diagnostic approaches, including direct detection methods, indirect serological techniques, and innovative point-of-care (POC) solutions. We examine the principles, advantages, and limitations of each method, with special focus on their clinical applicability in different healthcare settings.
Herpes is a widespread viral infection caused by the herpes simplex virus (HSV) with no cure. HSV, divided into two subtypes: HSV-1 (primarily causing oral herpes) and HSV-2 (primarily causing genital herpes), affects billions of people worldwide. While many infections are asymptomatic, HSV can cause painful ulcers, neonatal complications, and an increased risk of HIV transmission. Accurate and timely diagnosis is crucial to controlling the epidemic, preventing transmission, and improving patient outcomes.
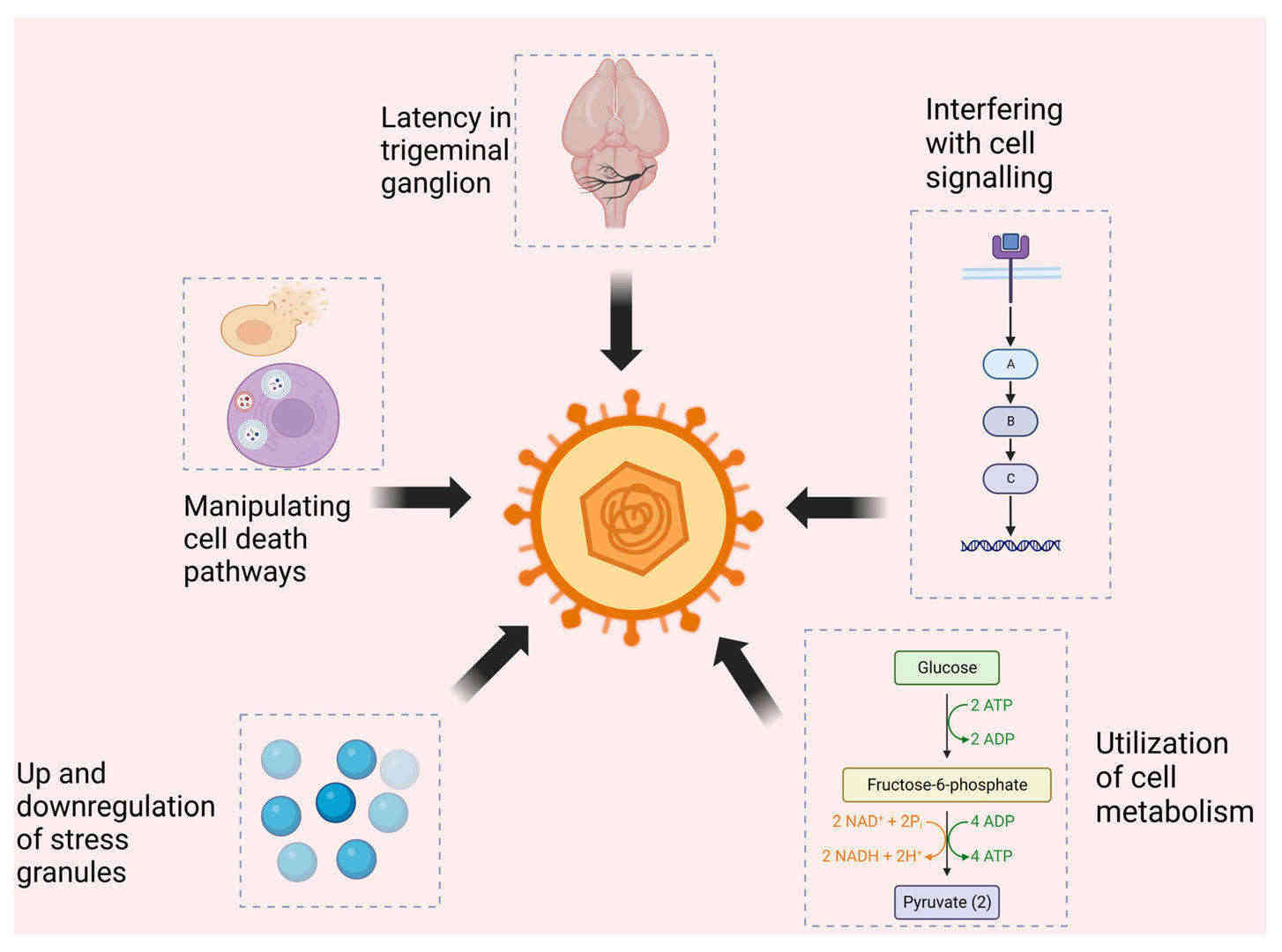 Fig.1 HSV-2's survival strategies and impact on host defense mechanisms. (Borase H, Shukla D., 2023)
Fig.1 HSV-2's survival strategies and impact on host defense mechanisms. (Borase H, Shukla D., 2023)
Direct detection methods identify herpes simplex virus (HSV) components (viral particles, proteins, or genetic material) in clinical samples, offering rapid and definitive diagnosis of active infection. These techniques are crucial for distinguishing HSV from other pathogens with similar symptoms. Below, we explore three key approaches:
Microscopy and Imaging
Microscopic techniques for HSV diagnosis focus on identifying virus-induced cytopathic effects in cells and tissues. The classic Tzanck smear examines lesion scrapings under light microscopy after staining, revealing pathognomonic findings like multinucleated giant cells and intranuclear inclusion bodies (Cowdry type A). While rapid and inexpensive, this method cannot distinguish HSV from VZV and has moderate sensitivity (50-70%).

Detection of Viral Glycoproteins
HSV glycoproteins (such as gB and gD) are key targets for antigenic diagnostic testing. Agglutination tests and Western blotting are two well-established methods.
Detection of Viral Genetic Material
Molecular techniques, particularly PCR, represent the gold standard for HSV detection due to their exceptional sensitivity (>95%) and ability to quantify viral load. PCR amplifies HSV DNA from swabs, cerebrospinal fluid (CSF), or blood, enabling diagnosis even in asymptomatic or low-shedding cases. Isothermal amplification methods (e.g., LAMP, RPA) further simplify nucleic acid testing, while next-generation sequencing (NGS) aids in strain typing for research.
Serological testing for HSV detects host antibodies (IgG/IgM) produced in response to infection, rather than the virus itself. These methods are particularly useful for identifying past exposure, differentiating HSV-1 from HSV-2, and screening asymptomatic individuals. While they cannot diagnose acute infections, serological assays play a key role in epidemiological studies and clinical management.

ELISA is the most common serological test for HSV, using enzyme-labeled antibodies to detect HSV-specific IgG/IgM in patient serum. The assay provides quantitative results with high throughput, making it ideal for large-scale screening. However, some commercial ELISAs may cross-react with other herpesviruses, requiring confirmation by more specific tests (e.g., Western blot).
FIA employs fluorophore-labeled antibodies to identify HSV antibodies, offering enhanced sensitivity over ELISA. Automated systems reduce human error and improve reproducibility. FIA is particularly useful for low-antibody titer samples but requires specialized equipment and trained personnel.

MFIA technology uses bead-based arrays and flow cytometry to simultaneously detect antibodies against multiple pathogens (e.g., HSV-1, HSV-2, VZV). This high-throughput approach significantly reduces processing time and sample volume requirements while maintaining excellent specificity.

LIPS is a high-sensitivity research tool that uses luciferase-tagged HSV antigens to detect antibodies via light emission. It excels in distinguishing HSV-1/2 with minimal cross-reactivity and is valuable for vaccine studies. However, its complexity limits routine clinical use.
Point-of-care (POC) testing for HSV has emerged as a critical diagnostic approach to overcome limitations of conventional laboratory-based methods, particularly in resource-limited settings and for rapid clinical decision-making. The following summarizes three key points in the preparation of the HSV detection POC device based on microfluidics technology:
Microfluidic platforms utilize channels (<1000 μm) made from polymers, paper, glass, or fibers to enable rapid reagent diffusion, laminar flow, and efficient small-volume sample processing.
Gold/fluorescent nanoparticle-labeled antibodies are embedded in nitrocellulose membranes to detect HSV antigens/antibodies (e.g., HSV-2 gG2), with results visualized via colored lines.
Devices are designed for minimal user steps: sample application (blood/serum), buffer-assisted flow through separation membranes, and visual readout within ~15 minutes, achieving high accuracy.
This resource provides a comprehensive overview of herpes simplex virus (HSV) diagnostic technologies, spanning direct detection methods (microscopy, antigen tests, and molecular assays) to indirect serological approaches (ELISA, immunoassays) and innovative point-of-care (POC) solutions. While traditional lab-based tests like PCR remain the gold standard for sensitivity and specificity, emerging microfluidics-based POC devices and rapid antigen/antibody tests are transforming HSV diagnosis by enabling faster, decentralized testing, which is critical for controlling transmission and complications.
Alta DiagnoTech offers a comprehensive portfolio of IVD solutions for accurate and timely diagnosis of herpes simplex virus (HSV) infections, spanning from high-precision lab-based tests to rapid point-of-care (POC) detection systems. If you have related needs, please feel free to contact us for more information or product support.
References
This article is for research use only. Do not use in any diagnostic or therapeutic application.
|
There is no product in your cart. |