- Home
- Resource
- Explore & Learn
- Graphene Oxide-Enabled Cancer Diagnostics: Machine Learning-Driven Drug Delivery and Analytical Strategies
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Graphene oxide (GO), a two-dimensional nanomaterial derived from graphene, has emerged as a revolutionary component in cancer research. Its unique structure, characterized by a single-atom-thick layer of carbon atoms with oxygen-containing functional groups attached, endows it with remarkable properties. The high surface-area-to-volume ratio of GO makes it an excellent carrier for drug delivery. For instance, in pre-clinical studies, GO has been successfully loaded with chemotherapy drugs such as doxorubicin. The functional groups on GO can be modified to target specific cancer cells. By attaching folic acid molecules, which are recognized by folate receptors overexpressed on many cancer cells, like ovarian cancer cells, the GO-drug conjugate can selectively accumulate at the tumor site, increasing the local drug concentration while reducing systemic toxicity.
GO also exhibits photothermal properties, making it a valuable tool for hyperthermia treatment. When exposed to near-infrared (NIR) light, GO can convert the light energy into heat, leading to the destruction of cancer cells. In animal models of breast cancer, NIR-mediated photothermal therapy using GO nanoparticles resulted in significant tumor shrinkage. The heat generated by GO can disrupt the cellular structure of cancer cells, including damaging cell membranes and denaturing proteins, ultimately causing cell death.
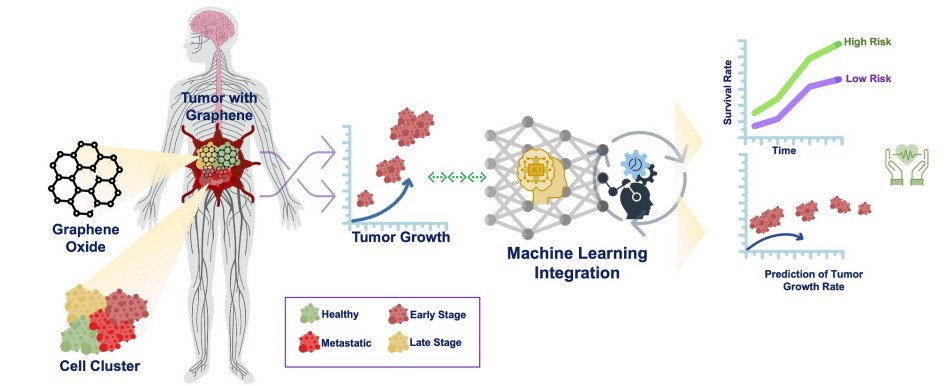 Fig.1 (a) Graphical representation of GO and rGO-assisted approaches for cancer management. GO, graphene oxide; rGO, reduced graphene oxide. (Das S., et al., 2025)
Fig.1 (a) Graphical representation of GO and rGO-assisted approaches for cancer management. GO, graphene oxide; rGO, reduced graphene oxide. (Das S., et al., 2025)

Machine learning (ML) algorithms have transformed the landscape of cancer diagnosis and prognosis. In the field of medical imaging, convolutional neural networks (CNNs), a type of deep learning algorithm, have shown outstanding performance. When applied to mammograms, CNNs can detect early-stage breast cancer with high accuracy. By analyzing thousands of mammogram images, these algorithms learn to identify subtle patterns associated with cancerous lesions. For example, a well-trained CNN can distinguish between benign and malignant microcalcifications, which are often early indicators of breast cancer.
ML also plays a crucial role in predicting cancer prognosis. Survival analysis models, such as Cox proportional-hazards models enhanced with ML techniques, can analyze patient-specific data, including age, tumor stage, genetic markers, and treatment history. In lung cancer patients, these models can predict the probability of recurrence and overall survival, enabling clinicians to tailor treatment plans more effectively. By incorporating genetic data, ML algorithms can identify patients who are more likely to respond to certain targeted therapies, improving treatment outcomes.
The combination of GO-based biosensors and ML has revolutionized cancer diagnosis. GO-based electronic nose (e-nose) systems are designed to detect volatile organic compounds (VOCs) in human breath, which can serve as cancer biomarkers. In a study on lung cancer, an e-nose system utilizing GO-functionalized sensors was able to detect lung cancer with a sensitivity of 95.8% and a specificity of 96.0%. The GO sensors have a high affinity for VOCs, and the electrical properties of GO change upon binding with these compounds.
ML algorithms, such as support vector machines (SVMs), are then employed to analyze the sensor data. SVMs can classify the complex patterns of electrical signals from the GO sensors, differentiating between breath samples of healthy individuals and those with lung cancer. This non-invasive diagnostic approach offers a faster and more convenient alternative to traditional invasive methods like lung biopsies.
In liquid biopsy applications, GO-based nanosensors are used to capture circulating tumor DNA (ctDNA) and exosomes. These nanosensors can selectively bind to ctDNA or exosomes due to the functionalization of GO with specific DNA probes or antibodies. ML algorithms are used to analyze the captured genetic material. For example, in colorectal cancer, ML-based analysis of ctDNA captured by GO-based nanosensors can detect the presence of cancer-associated mutations with high sensitivity, enabling early detection and monitoring of disease progression.
Machine learning can optimize GO-mediated drug delivery systems. Random forest algorithms, for instance, can predict the drug-loading efficiency of GO based on various factors such as the type of drug, the surface properties of GO, and the environmental conditions. By analyzing a large dataset of drug-GO interactions, these algorithms can identify the optimal conditions for maximum drug loading.
In pH-sensitive GO-based drug delivery systems, ML can predict the release kinetics of drugs in different physiological environments. Tumor tissues typically have a lower pH compared to normal tissues. GO-based nanoparticles can be designed to release drugs in response to this acidic environment. ML models can simulate how these nanoparticles will behave in various pH conditions within the body, helping researchers to fine-tune the design of the drug delivery system. In a study on gastric cancer, ML-optimized GO-based drug delivery systems showed enhanced drug release at the tumor site, resulting in more effective tumor suppression compared to non-optimized systems.
Despite the significant progress, several challenges remain in the integration of GO and ML in cancer treatment. One major challenge is the large-scale production of high-quality GO with consistent properties. Variations in GO synthesis can lead to differences in its performance in drug delivery and sensing applications. Standardized production methods need to be developed to ensure reproducibility.
Another challenge is the interpretability of complex ML models. Deep learning algorithms, in particular, are often considered "black boxes," making it difficult for clinicians to understand how they arrive at their predictions. Efforts are underway to develop techniques for model interpretability, such as generating feature importance maps for CNNs used in cancer diagnosis.
In the future, the combination of GO and ML is likely to expand. Multimodal sensors that combine GO with other sensing technologies, such as Raman spectroscopy, may be developed to detect multiple cancer biomarkers simultaneously. This will provide more comprehensive information for cancer diagnosis and treatment monitoring. Additionally, the integration of GO-ML systems with the Internet of Things (IoT) could enable real-time, remote monitoring of cancer patients, facilitating personalized and continuous care.
If you have related needs, please feel free to contact us for more information or product support.
Reference
This article is for research use only. Do not use in any diagnostic or therapeutic application.

Cat.No. GP-DQL-00203
Rotavirus Antigen Group A and Adenovirus Antigen Rapid Test Kit (Colloidal Gold)

Cat.No. GP-DQL-00206
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Card Style

Cat.No. GP-DQL-00207
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. GP-DQL-00211
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. GP-DQL-00212
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. IP-00189
Influenza A Rapid Assay Kit

Cat.No. GH-DQL-00200
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold)
Cat.No. GH-DQL-00201
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00202
Luteinizing Hormone Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00208
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold), Strip Style
Cat.No. GH-DQL-00209
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit(Colloidal Gold), Strip Style

Cat.No. GH-DQL-00210
Luteinizing Hormone Rapid Test Kit (Colloidal Gold), Strip Style
Cat.No. IH-HYW-0001
hCG Pregnancy Test Strip
Cat.No. IH-HYW-0002
hCG Pregnancy Test Cassette
Cat.No. IH-HYW-0003
hCG Pregnancy Test Midstream

Cat.No. GD-QCY-0001
Cocaine (COC) Rapid Test Kit

Cat.No. GD-QCY-0002
Marijuana (THC) Rapid Test Kit
Cat.No. GD-QCY-0003
Morphine (MOP300) Rapid Test Kit
Cat.No. GD-QCY-0004
Methamphetamine (MET) Rapid Test Kit
Cat.No. GD-QCY-0005
Methylenedioxymethamphetamine ecstasy (MDMA) Rapid Test Kit
Cat.No. GD-QCY-0006
Amphetamine (AMP) Rapid Test Kit
Cat.No. GD-QCY-0007
Barbiturates (BAR) Rapid Test Kit

Cat.No. GD-QCY-0008
Benzodiazepines (BZO) Rapid Test Kit
Cat.No. GD-QCY-0009
Methadone (MTD) Rapid Test Kit
Cat.No. GD-QCY-0011
Opiate (OPI) Rapid Test Kit
Cat.No. ID-HYW-0002
Multi-Drug Test L-Cup, (5-16 Para)
Cat.No. ID-HYW-0005
Multi-Drug Rapid Test (Dipcard & Cup) with Fentanyl
Cat.No. ID-HYW-0006
Multi-Drug Rapid Test (Dipcard & Cup) without Fentanyl
Cat.No. ID-HYW-0007
Multi-Drug 2~14 Drugs Rapid Test (Dipstick & Dipcard & Cup)
Cat.No. ID-HYW-0008
Fentanyl (FYL) Rapid Test (For Prescription Use)
Cat.No. ID-HYW-0009
Fentanyl Urine Test Cassette (CLIA Waived)
Cat.No. ID-HYW-0010
Fentanyl Urine Test Cassette (Home Use)
|
There is no product in your cart. |