- Home
- Resource
- Explore & Learn
- Functional Antibodies-Based Ultrasensitive Electrochemical Immunosensor for Multiplex Sandwich Bioassays
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
In vitro diagnostics (IVD) are crucial in modern healthcare, offering early disease detection, prognosis, and monitoring of treatment responses. As the healthcare sector moves toward personalized medicine, the need for sensitive, specific, and real-time diagnostic technologies has never been greater. One of the most promising advancements in IVD is the development of electrochemical immunosensors, devices capable of detecting specific biomarkers with remarkable precision and speed. These sensors play a pivotal role in the diagnosis of diseases such as cancer, cardiovascular conditions, and autoimmune disorders, among others.
Electrochemical immunosensors represent a significant departure from traditional diagnostic tools by directly measuring the electrical signals generated by a chemical reaction. In recent years, significant strides have been made to enhance their sensitivity, specificity, and multiplexing capabilities, making them indispensable for clinical diagnostics. The integration of functional antibodies and thionine (Thi)-based labeling in these sensors has proven to be a breakthrough in overcoming the limitations of earlier systems.
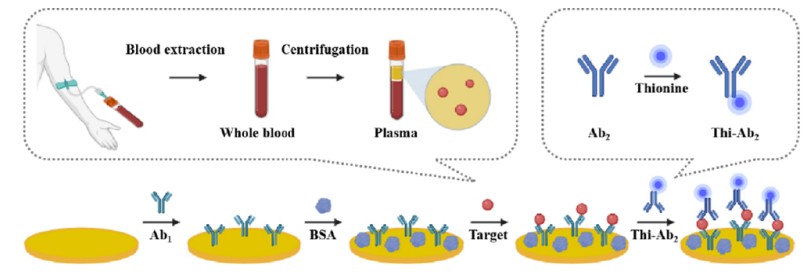 Fig.1 Schematic illustration of the fabrication process of Thi-Ab2 and electrochemical immunosensor. (Liu L., et al., 2024)
Fig.1 Schematic illustration of the fabrication process of Thi-Ab2 and electrochemical immunosensor. (Liu L., et al., 2024)
Electrochemical immunosensors are devices that combine the principles of electrochemistry and immunology to detect specific analytes—such as proteins, hormones, or nucleic acids—in biological samples. These sensors typically consist of an electrochemical transducer and antibodies that selectively bind to the target analytes. The transducer detects the electrochemical signal produced by the interaction between the target and the antibody, which can then be quantified.
These devices are based on the sandwich assay format, wherein capture antibodies (Ab1) immobilize the target biomarker, followed by the addition of detection antibodies (Ab2), which are often labeled with electroactive tags such as thionine. When the analyte is present, it triggers a measurable redox reaction, producing a distinct electrochemical signal that can be analyzed.

Electrochemical immunosensors are highly favored for in vitro diagnostics due to their simplicity, cost-effectiveness, and ability to provide real-time results. Traditional diagnostic techniques, such as enzyme-linked immunosorbent assays (ELISA) or immunofluorescence, can be time-consuming and require sophisticated equipment and skilled personnel. Electrochemical immunosensors, in contrast, are portable, easy to use, and suitable for point-of-care testing.
Moreover, the high sensitivity of electrochemical immunosensors allows for the detection of analytes at ultralow concentrations (down to femtogram/milliliter levels), which is crucial for the early detection of diseases before clinical symptoms arise. The specificity of these sensors ensures that the signal generated is primarily from the target biomarker, minimizing interference from other substances in the sample.
A significant enhancement in electrochemical immunosensor performance comes from the use of thionine (Thi), an electrochemical label that is used to modify detection antibodies (Ab2). When conjugated with antibodies, Thi serves as an electrochemical tag that produces a distinct redox signal when exposed to an applied voltage. This signal can be easily detected and quantified, making it ideal for use in highly sensitive immunoassays.
Thionine-modified antibodies have several advantages over traditional labeling techniques, such as enzymatic or fluorescent tags. The use of Thi allows for a direct electrochemical readout, eliminating the need for additional steps like substrate reactions in enzyme-based assays, which can complicate the process and reduce reliability. Furthermore, Thi's strong electrochemical properties enhance the signal intensity, enabling the detection of extremely low concentrations of target analytes.
The process begins with the synthesis of a Thi-Ab2 composite, where Ab2 is first modified using a coupling agent (e.g., EDC/NHS) to activate the carboxyl groups on the antibody. The Thi molecules are then covalently bound to the antibody through amide bond formation. This composite is stable and retains its binding affinity to the target protein.
Once the modified antibodies (Thi-Ab2) are introduced into the detection system, they bind to the target analyte (e.g., TNF-alpha, cardiac troponin I, or interleukin-6) in a sandwich configuration. The binding results in the formation of a complex that generates a detectable electrochemical signal when the electrode is subjected to a voltage. This system allows for the quantification of protein biomarkers even at very low concentrations, with limits of detection (LoDs) in the femtogram range.
One of the most compelling features of electrochemical immunosensors is their sensitivity. The Thi-based electrochemical immunosensor is capable of detecting biomarkers at concentrations as low as 9.38 fg/mL for TNF-alpha, 1.70 fg/mL for cardiac troponin I (cTnI), and 8.14 fg/mL for interleukin-6 (IL-6). These limits of detection are significantly lower than those achievable with many traditional diagnostic methods.
The ultrasensitive detection capability is especially important in clinical diagnostics, where early-stage detection of diseases like cancer or cardiovascular conditions can significantly improve patient outcomes. For instance, the ability to detect TNF-alpha, a critical biomarker for inflammation and autoimmune disorders, at such low levels can be invaluable in monitoring disease progression and response to treatment.

Another key challenge in diagnostics is specificity—ensuring that the sensor responds only to the target analyte and not to interfering substances in the sample. The Thi-based electrochemical immunosensor has shown exceptional specificity, capable of distinguishing between closely related biomarkers and minimizing interference from nontarget proteins like SARS-CoV-2, influenza, and BSA. This high specificity is crucial for ensuring accurate diagnosis, particularly when dealing with complex biological samples that may contain a variety of proteins with similar structural features.
In the context of disease diagnostics, being able to measure multiple biomarkers simultaneously—multiplexing—can provide a more comprehensive understanding of a patient's condition. Electrochemical immunosensors are ideally suited for multiplexing, as they can be modified to simultaneously detect several biomarkers using a multi-electrode system.
By modifying the sensor to detect proteins like cTnI and IL-6 at the same time, clinicians can gain a broader picture of a patient's health status. For example, cTnI is a key biomarker for heart attacks, while IL-6 is associated with inflammatory processes. Having the ability to detect both biomarkers in a single test can provide more accurate and efficient disease monitoring.
The electrochemical immunosensor has proven to be effective not only for single biomarker detection but also for multiplexed protein detection. In tests, the limits of detection for cTnI and IL-6 were 1.70 fg/mL and 8.14 fg/mL, respectively, in a multiplex format. This high sensitivity across multiple targets enhances the sensor's diagnostic utility, allowing for the simultaneous monitoring of multiple disease markers, thereby improving the accuracy and speed of clinical decision-making.
The clinical applicability of electrochemical immunosensors was demonstrated by evaluating their performance in human serum samples. The sensors were able to detect TNF-alpha in serum with a limit of detection of 70.44 fg/mL, even when the sample was diluted to just 30%. This demonstrates the sensor's ability to perform well in real-world, complex biological samples, where interference from other proteins and substances can be a significant challenge.
Moreover, the repeatability of the immunosensor, with low coefficients of variation (CVs) across multiple runs, underscores its potential for reliable, high-throughput diagnostics. The ability to detect ultra-low concentrations of target biomarkers in complex samples positions electrochemical immunosensors as a powerful tool for clinical diagnostics, including early-stage disease detection and monitoring of chronic conditions.
The development of electrochemical immunosensors is also paving the way for the miniaturization and automation of diagnostic tests. These sensors can be integrated into portable, point-of-care devices that can provide real-time results at the patient's bedside, reducing the need for centralized laboratories. This rapid diagnostic capability can be especially crucial in emergency settings, where immediate diagnosis is required to guide treatment decisions.
The advent of electrochemical immunosensors equipped with thionine-based labeling has marked a significant advancement in disease diagnosis. These sensors offer unparalleled sensitivity, specificity, and multiplexing capabilities, making them an invaluable tool in the evolving field of precision medicine. With the ability to detect biomarkers at extremely low concentrations in complex samples, electrochemical immunosensors hold the promise of revolutionizing early disease detection and personalized treatment strategies.
As the technology continues to evolve, the integration of these sensors into point-of-care platforms will further enhance their accessibility and utility, offering a future where disease diagnosis is faster, more accurate, and more tailored to individual patient needs. The ongoing advancements in electrochemical immunosensors represent a major step forward in improving healthcare outcomes globally, moving us closer to a world of real-time, targeted diagnostics.
If you have related needs, please feel free to contact us for more information or product support.
Reference
This article is for research use only. Do not use in any diagnostic or therapeutic application.

Cat.No. GP-DQL-00203
Rotavirus Antigen Group A and Adenovirus Antigen Rapid Test Kit (Colloidal Gold)

Cat.No. GP-DQL-00206
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Card Style

Cat.No. GP-DQL-00207
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. GP-DQL-00211
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. GP-DQL-00212
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. IP-00189
Influenza A Rapid Assay Kit

Cat.No. GH-DQL-00200
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00201
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00202
Luteinizing Hormone Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00208
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. GH-DQL-00209
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit(Colloidal Gold), Strip Style

Cat.No. GH-DQL-00210
Luteinizing Hormone Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. IH-HYW-0001
hCG Pregnancy Test Strip

Cat.No. IH-HYW-0002
hCG Pregnancy Test Cassette

Cat.No. IH-HYW-0003
hCG Pregnancy Test Midstream

Cat.No. GD-QCY-0001
Cocaine (COC) Rapid Test Kit

Cat.No. GD-QCY-0002
Marijuana (THC) Rapid Test Kit

Cat.No. GD-QCY-0003
Morphine (MOP300) Rapid Test Kit

Cat.No. GD-QCY-0004
Methamphetamine (MET) Rapid Test Kit

Cat.No. GD-QCY-0005
Methylenedioxymethamphetamine ecstasy (MDMA) Rapid Test Kit

Cat.No. GD-QCY-0006
Amphetamine (AMP) Rapid Test Kit

Cat.No. GD-QCY-0007
Barbiturates (BAR) Rapid Test Kit

Cat.No. GD-QCY-0008
Benzodiazepines (BZO) Rapid Test Kit

Cat.No. GD-QCY-0009
Methadone (MTD) Rapid Test Kit

Cat.No. GD-QCY-0011
Opiate (OPI) Rapid Test Kit

Cat.No. ID-HYW-0002
Multi-Drug Test L-Cup, (5-16 Para)

Cat.No. ID-HYW-0005
Multi-Drug Rapid Test (Dipcard & Cup) with Fentanyl

Cat.No. ID-HYW-0006
Multi-Drug Rapid Test (Dipcard & Cup) without Fentanyl

Cat.No. ID-HYW-0007
Multi-Drug 2~14 Drugs Rapid Test (Dipstick & Dipcard & Cup)

Cat.No. ID-HYW-0008
Fentanyl (FYL) Rapid Test (For Prescription Use)

Cat.No. ID-HYW-0009
Fentanyl Urine Test Cassette (CLIA Waived)

Cat.No. ID-HYW-0010
Fentanyl Urine Test Cassette (Home Use)
|
There is no product in your cart. |