- Home
- Resource
- Disease Diagnosis
- Infectious Diseases
- From Clinical Suspicion to Confirmation: Navigating the Diagnostic Path for Typhoid Fever
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Typhoid fever is a serious systemic bacterial infection that requires precise laboratory confirmation for accurate diagnosis and effective treatment. This resource provides a clear, step-by-step guide through the diagnostic pathway for typhoid fever, detailing the journey from initial clinical suspicion and evaluation, through the use of gold-standard and advanced diagnostic methods, to the critical final stages of confirmation, antimicrobial susceptibility testing, and carrier detection.
Typhoid fever is a life-threatening systemic infection caused by the bacterium Salmonella enterica serotype Typhi. It is primarily transmitted through the fecal-oral route via contaminated food or water in areas with poor sanitation. The disease is characterized by a prolonged, high-grade fever often accompanied by non-specific symptoms such as headache, malaise, abdominal pain, and constipation or diarrhea. Diagnosis is challenging due to its clinical overlap with other febrile illnesses and relies on definitive laboratory confirmation, most commonly through blood culture (the gold standard), with supportive roles for bone marrow culture, stool culture, and increasingly, molecular methods like PCR. Without prompt antibiotic treatment, complications such as intestinal perforation or hemorrhage can occur.
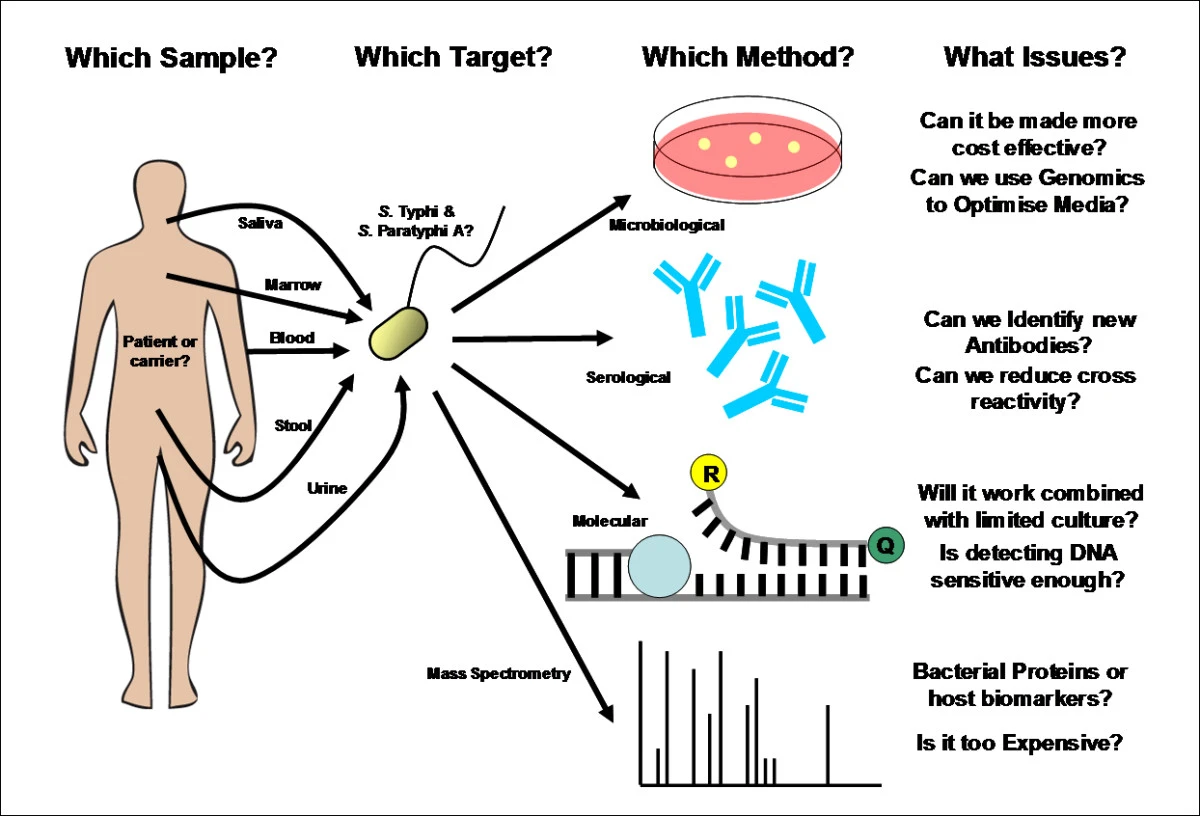 Fig.1 Identifying the techniques and issues which surround the development of a new enteric fever diagnostic test. Samples, targets, methods and issues. (Baker, Stephen, et al., 2010)
Fig.1 Identifying the techniques and issues which surround the development of a new enteric fever diagnostic test. Samples, targets, methods and issues. (Baker, Stephen, et al., 2010)
Clinical suspicion for typhoid fever should arise when a patient presents with a prolonged, high-grade fever, especially if accompanied by non-specific symptoms such as headache, malaise, abdominal discomfort, and relative bradycardia (a pulse rate slower than expected for the degree of fever). This suspicion is significantly heightened by key epidemiological risk factors, most importantly a history of recent travel to or residence in an endemic region (parts of South Asia, Southeast Asia, Africa, Latin America) and potential exposure to contaminated food or water. In this context, typhoid fever must be actively considered and ruled out through laboratory testing, as its early symptoms closely mimic other common febrile illnesses like malaria, dengue, and rickettsial infections.
The initial evaluation of suspected typhoid fever involves critical first-line laboratory investigations that serve to both support the clinical suspicion and provide a definitive diagnosis. This stage focuses on identifying characteristic, though non-specific, patterns in routine blood work and obtaining the gold-standard microbiological confirmation.

Routine laboratory findings in typhoid fever often reveal characteristic but non-specific patterns, most notably leukopenia (a low white blood cell count) and neutropenia. Other common findings include mild anemia, thrombocytopenia, and elevated liver transaminases. While these results are not diagnostic on their own, they strongly support the clinical suspicion of a systemic bacterial infection like typhoid and help narrow the differential diagnosis.

Blood culture is the definitive gold-standard test for confirming typhoid fever. Its sensitivity is highest (up to 80%) during the first week of illness and before antibiotic administration. A positive culture not only confirms the presence of Salmonella Typhi but is crucial for performing antimicrobial susceptibility testing (AST), which is essential for guiding effective antibiotic therapy in an era of increasing multidrug resistance.
When initial blood cultures are negative, inconclusive, or results are delayed, advanced diagnostic methods play a vital role in the evaluation of suspected typhoid fever. These techniques, primarily serologic tests and molecular diagnostics, offer alternative pathways for supporting the diagnosis, though they differ significantly in their principles, performance, and clinical interpretation.
Serologic Tests
Serologic tests, such as the traditional Widal test, detect antibodies produced in response to Salmonella Typhi antigens (O and H). While widely available, they have significant limitations, including a lack of standardized interpretation, potential for cross-reactivity with other infections or vaccines, and the requirement for paired acute and convalescent serum samples to demonstrate a rising antibody titer. Consequently, a single positive result is not diagnostic and these tests should only be used as supplementary evidence in conjunction with clinical findings and culture.
Molecular Diagnostics
Molecular diagnostics, primarily nucleic acid amplification tests (NAATs) like PCR, detect specific genetic material (DNA) of Salmonella Typhi directly in patient samples such as blood. These tests offer high specificity and faster results compared to culture and can be valuable for patients who have already started antibiotics, which may inhibit bacterial growth in culture. A key limitation is that a positive NAAT indicates the presence of the bacterium but does not provide data on antimicrobial susceptibility, which is critical for guiding treatment.
Following the diagnosis of typhoid fever, the focus shifts from detection to precise management and public health action. This critical phase involves definitively confirming the infection, determining the most effective antibiotic treatment, and identifying individuals who may unknowingly spread the bacteria.
To address the critical need for accurate and timely diagnosis of typhoid fever, Alta DiagnoTech provides a comprehensive portfolio of in vitro diagnostic (IVD) solutions. Our offerings support the complete diagnostic journey, from initial culture-based confirmation to rapid molecular detection and antimicrobial resistance profiling, empowering laboratories to deliver precise results essential for effective patient management and public health surveillance. If you have related needs, please feel free to contact us for more information or product support.
| Product Name | Technology | Application |
| Automated Blood Culture System & Media | Automated, Continuous-Monitoring Culture System | Enrichment and detection of Salmonella Typhi and other bloodstream pathogens from patient blood samples, serving as the foundational step for isolation and definitive diagnosis. |
| Salmonella Serogroup & Serotype Agglutination Sera | Slide/Tube Agglutination Test | Presumptive identification and serological typing of Salmonella isolates (including Typhi) recovered from culture, crucial for epidemiological tracking. |
| Typhoid Fever Multiplex PCR Detection Kit | Multiplex Real-Time PCR | Simultaneous, rapid, and specific detection of Salmonella Typhi DNA and genes associated with common antibiotic resistance (e.g., to fluoroquinolones) directly from blood or stool samples. |
| Automated Antimicrobial Susceptibility Testing (AST) System & Panels | Broth Microdilution / Disk Diffusion | Determination of the antibiotic susceptibility profile of cultured Salmonella Typhi isolates to guide targeted and effective antimicrobial therapy. |
| Stool Culture Medium for Enteric Pathogens | Selective Agar Culture | Isolation and identification of Salmonella Typhi from stool samples, primarily used for the detection of convalescent carriers or in chronic carrier screening programs. |
Reference
This article is for research use only. Do not use in any diagnostic or therapeutic application.
|
There is no product in your cart. |