- Home
- Resource
- Explore & Learn
- Examining Viability as an Indicator for Assessing Lymphocyte Transformation in Drug Hypersensitivity Diagnosis
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Drug hypersensitivity reactions are a significant concern in modern medicine, affecting a notable portion of the population and posing challenges for both patients and healthcare providers. These reactions are immune-mediated adverse responses to medications, often leading to unpredictable and potentially severe outcomes. Traditional diagnostic methods, such as clinical history assessment, skin testing, and in vitro tests like the Lymphocyte Transformation Test (LTT), have been the cornerstone of drug allergy diagnosis. However, these methods often fall short due to limitations in sensitivity, specificity, and the ability to replicate in vivo conditions accurately. Recent advancements in in vitro diagnostics, particularly the use of cell viability tests, offer a promising new approach to overcoming these challenges.
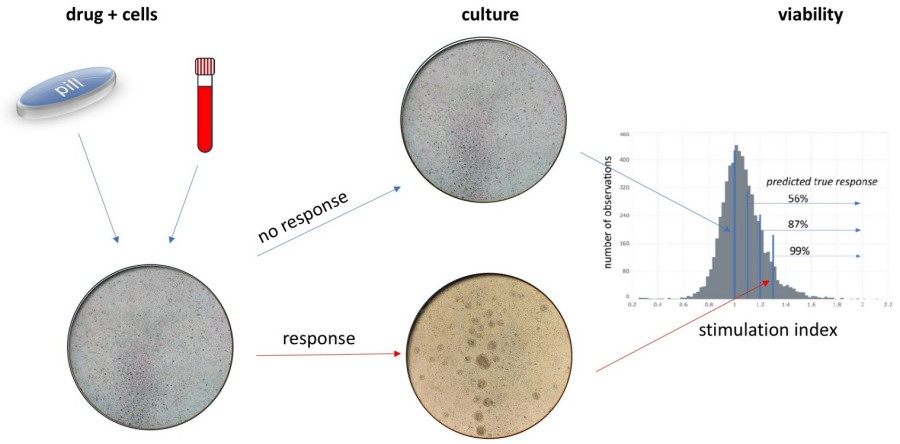 Fig.1 In vitro tests of cellular activity. (Gyovai A., et al., 2025)
Fig.1 In vitro tests of cellular activity. (Gyovai A., et al., 2025)
In vitro diagnostics have long been an essential tool in the arsenal of allergists and immunologists. Traditional methods, such as the LTT, measure lymphocyte proliferation in response to drug exposure. However, these tests often rely on radioactive materials and have limited sensitivity, making them less than ideal for widespread use. The introduction of viability-based assays has revolutionized this field by providing a non-radioactive, highly sensitive alternative. These assays measure the metabolic activity of cells, offering a comprehensive readout that includes both proliferation and cell death signals. This dual measurement capability enhances the diagnostic accuracy and reliability of in vitro tests.
Viability-based Lymphocyte Transformation Tests (LTTs) leverage the metabolic activity of lymphocytes to assess their response to drug exposure. Unlike traditional LTTs that measure proliferation through radioactive thymidine incorporation, viability-based assays use colorimetric or fluorometric methods to quantify cell survival. The MTT assay, for example, measures the reduction of a tetrazolium salt to formazan crystals by metabolically active cells. This method provides a quantitative measure of cell viability, which can be used to calculate a stimulation index (SI) indicative of the immune response to a drug.
The SI is calculated as the ratio of the signal from treated cells to that from untreated controls. An SI greater than a predefined threshold (typically 1.4) indicates a positive response, suggesting hypersensitivity to the drug. This approach not only enhances sensitivity but also reduces the variability associated with traditional methods. The use of multiple parallel measurements further improves the statistical robustness of the results, allowing for more confident diagnosis.


Age and gender can significantly influence immune responses, and their impact on drug allergy testing is an important consideration. Studies have shown that the variance in negative control measurements remains consistent across different age groups, indicating that viability-based LTTs are applicable throughout adulthood. However, the responsiveness to mitogens, such as phytohemagglutinin (PHA), decreases with age, highlighting the need to account for age-related changes in immune function.
Gender differences in immune responses are also well-documented, with women generally exhibiting higher immune reactivity than men. This difference is reflected in the incidence of drug allergies, with women being more likely to develop adverse reactions. Despite these differences, the coefficient of variation (CV) in negative control measurements is statistically identical between men and women, suggesting that viability-based LTTs perform consistently across genders.
The analytical sensitivity and specificity of a diagnostic test are critical factors in its clinical utility. Viability-based LTTs have demonstrated high analytical specificity, with 99% of measurements above the lower cut-off value predicted to be true cellular responses. This high specificity is attributed to the low variability of the method when an adequate number of parallel measurements are used.
However, the diagnostic sensitivity of these tests is inherently limited by the inability of in vitro assays to fully replicate in vivo conditions. While the tests can detect cellular responses with high confidence, they may not capture all aspects of the immune response, particularly those dominated by cytotoxic T cells. Therefore, while viability-based LTTs are valuable for confirming hypersensitivity, they should not be used to exclude drug sensitivity.
Viability-based LTTs offer several practical advantages over traditional methods. They are non-radioactive, reducing the need for specialized handling and disposal procedures. Their high sensitivity and specificity make them suitable for detecting even modest increases in cellular responses, providing a more nuanced understanding of drug-induced hypersensitivity.
In clinical practice, these tests can be used to confirm the immunological basis of drug sensitivity, supporting clinical decision-making and patient management. They can also be combined with other in vitro allergy tests, such as basophil activation tests (BATs), to broaden the range of detectable immunological mechanisms. This multimodal approach can enhance diagnostic sensitivity and provide a more comprehensive assessment of drug allergy.
Table 1: Comparison of Traditional and Viability-Based LTTs. (Gyovai A., et al., 2025)
| Feature | Traditional LTT (Radioactive) | Viability-Based LTT (Non-Radioactive) |
| Measurement Method | Radioactive thymidine incorporation | MTT assay, colorimetric/fluorometric methods |
| Sensitivity | Moderate | High |
| Specificity | High | High |
| Safety | Requires specialized handling | Non-radioactive, safer to use |
| Statistical Robustness | Moderate | High |
| Clinical Utility | Limited by sensitivity | Enhanced diagnostic accuracy |
The field of drug allergy testing is continually evolving, driven by advancements in technology and a deeper understanding of immunological mechanisms. Viability-based LTTs represent a significant step forward, offering improved sensitivity and specificity over traditional methods. However, ongoing research is focused on further refining these assays and exploring new biomarkers and techniques to enhance diagnostic accuracy.
Future innovations may include the development of multiplex assays capable of simultaneously testing multiple drugs, reducing the need for repeated testing. Advances in imaging and molecular techniques may also provide new insights into the cellular and molecular mechanisms underlying drug hypersensitivity, leading to more targeted and personalized diagnostic approaches.
Viability-based Lymphocyte Transformation Tests represent a significant advancement in the field of drug allergy diagnostics. By providing a highly sensitive and specific measure of cellular responses, these assays offer a powerful tool for confirming drug hypersensitivity and supporting clinical decision-making. As we continue to refine and expand these methods, the future of drug allergy testing looks promising, with the potential to improve patient outcomes and enhance our understanding of these complex immune reactions.
If you have related needs, please feel free to contact us for more information or product support.
Reference
This article is for research use only. Do not use in any diagnostic or therapeutic application.

Cat.No. GP-DQL-00203
Rotavirus Antigen Group A and Adenovirus Antigen Rapid Test Kit (Colloidal Gold)

Cat.No. GP-DQL-00206
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Card Style

Cat.No. GP-DQL-00207
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. GP-DQL-00211
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. GP-DQL-00212
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. IP-00189
Influenza A Rapid Assay Kit

Cat.No. GH-DQL-00200
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00201
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00202
Luteinizing Hormone Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00208
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. GH-DQL-00209
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit(Colloidal Gold), Strip Style

Cat.No. GH-DQL-00210
Luteinizing Hormone Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. IH-HYW-0001
hCG Pregnancy Test Strip

Cat.No. IH-HYW-0002
hCG Pregnancy Test Cassette

Cat.No. IH-HYW-0003
hCG Pregnancy Test Midstream

Cat.No. GD-QCY-0001
Cocaine (COC) Rapid Test Kit

Cat.No. GD-QCY-0002
Marijuana (THC) Rapid Test Kit

Cat.No. GD-QCY-0003
Morphine (MOP300) Rapid Test Kit

Cat.No. GD-QCY-0004
Methamphetamine (MET) Rapid Test Kit

Cat.No. GD-QCY-0005
Methylenedioxymethamphetamine ecstasy (MDMA) Rapid Test Kit

Cat.No. GD-QCY-0006
Amphetamine (AMP) Rapid Test Kit

Cat.No. GD-QCY-0007
Barbiturates (BAR) Rapid Test Kit

Cat.No. GD-QCY-0008
Benzodiazepines (BZO) Rapid Test Kit

Cat.No. GD-QCY-0009
Methadone (MTD) Rapid Test Kit

Cat.No. GD-QCY-0011
Opiate (OPI) Rapid Test Kit

Cat.No. ID-HYW-0002
Multi-Drug Test L-Cup, (5-16 Para)

Cat.No. ID-HYW-0005
Multi-Drug Rapid Test (Dipcard & Cup) with Fentanyl

Cat.No. ID-HYW-0006
Multi-Drug Rapid Test (Dipcard & Cup) without Fentanyl

Cat.No. ID-HYW-0007
Multi-Drug 2~14 Drugs Rapid Test (Dipstick & Dipcard & Cup)

Cat.No. ID-HYW-0008
Fentanyl (FYL) Rapid Test (For Prescription Use)

Cat.No. ID-HYW-0009
Fentanyl Urine Test Cassette (CLIA Waived)

Cat.No. ID-HYW-0010
Fentanyl Urine Test Cassette (Home Use)
|
There is no product in your cart. |