- Home
- Resource
- Disease Diagnosis
- Cancers
- Enhancing Diagnostic Confidence: A Resource on Methods and Biomarkers for Accurate Melanoma Detection
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Melanoma is an aggressive form of skin cancer with significant metastatic potential, making its early and accurate detection critical for patient survival. This resource provides a comprehensive overview of the modern diagnostic landscape for melanoma, detailing the integrated clinical workflow from initial patient assessment to definitive biopsy. It further explores essential laboratory techniques, including histopathology and immunohistochemistry, and highlights the pivotal role of molecular biomarkers and genomic profiling in enabling precise diagnosis, prognostic stratification, and guiding targeted therapeutic decisions.
Melanoma is an aggressive form of skin cancer originating from melanocytes, the pigment-producing cells responsible for skin coloration. While it accounts for a minority of skin cancer cases, it is responsible for the majority of skin cancer-related deaths due to its high potential for metastasis if not detected early. Primary risk factors include intense, intermittent ultraviolet (UV) radiation exposure, a history of sunburns, fair skin, and genetic predisposition. Clinically, melanoma often presents as a changing mole, and its diagnosis relies on a combination of clinical examination, dermoscopy, and histopathological analysis of biopsied tissue. Early and accurate detection is paramount, as localized disease is highly curable with surgical excision, while advanced stages have a significantly poorer prognosis. This underscores the critical need for refined diagnostic methodologies and robust biomarker development to improve patient outcomes.
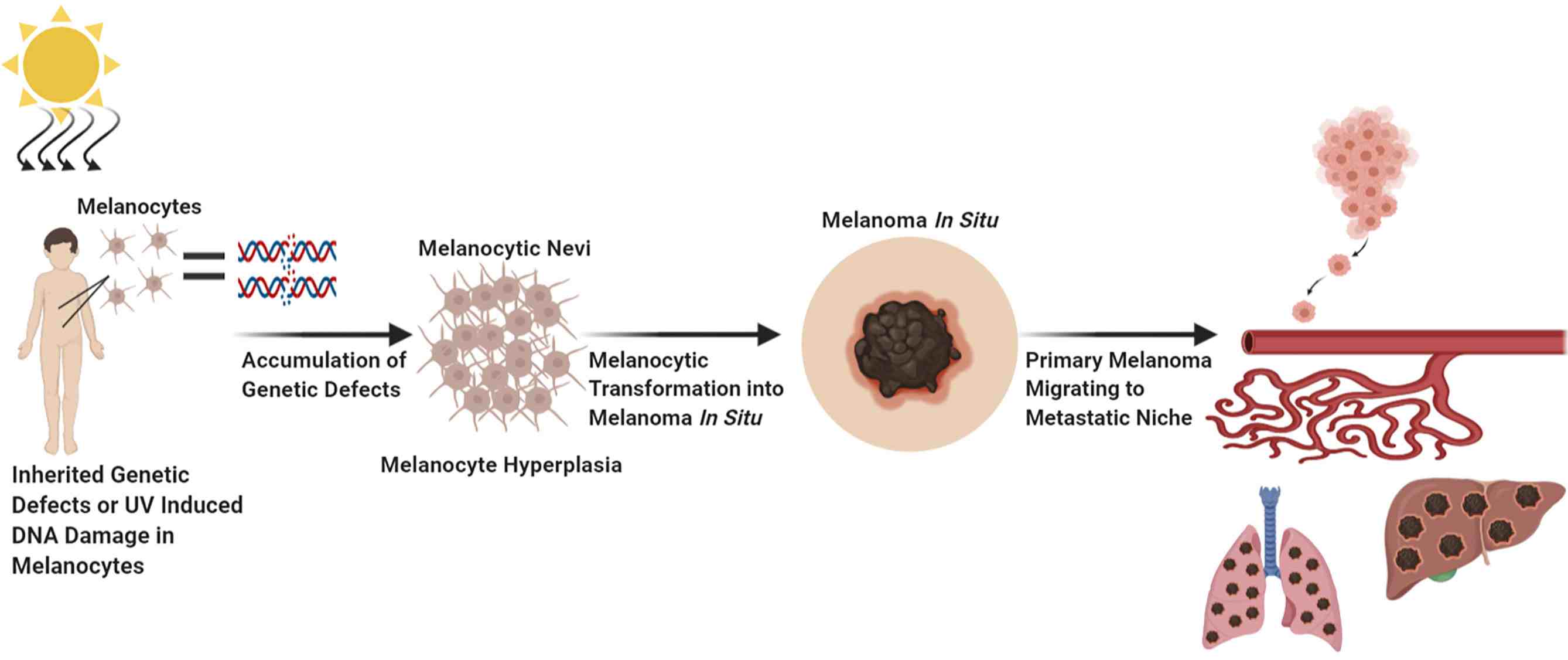 Fig.1 Factors which contribute to melanocytic transformation. (Eddy K, et al., 2021)
Fig.1 Factors which contribute to melanocytic transformation. (Eddy K, et al., 2021)
The clinical diagnosis of melanoma follows a structured, multi-step pathway designed to identify suspicious lesions with high accuracy while minimizing unnecessary procedures. This process integrates initial risk assessment, enhanced visual analysis, and a definitive decision to proceed with tissue sampling, forming a critical bridge between suspicion and pathological confirmation.

The process begins with a visual skin exam using the "ABCDE" rule and an assessment of key risk factors, including UV exposure history, skin type, and family history of melanoma. This step stratifies patient risk and identifies lesions requiring closer scrutiny.

UDermoscopy provides a magnified, detailed view of subsurface skin structures using a handheld device. This tool significantly improves diagnostic accuracy by revealing patterns and microstructures not visible to the naked eye, helping differentiate early melanomas from benign lesions.

A biopsy is performed when a lesion shows clear clinical and dermoscopic features of melanoma. This decisive step provides the tissue sample required for definitive histopathological diagnosis, guiding all subsequent treatment decisions.
While clinical assessment identifies suspicious lesions, definitive diagnosis relies on specialized laboratory techniques that analyze tissue at the cellular and molecular level. These methods provide the critical evidence needed to confirm melanoma, subtype it, and gather prognostic information, forming the cornerstone of all subsequent treatment decisions. The most pivotal of these techniques are histopathology and immunohistochemistry.
Histopathology: The Gold Standard
Histopathological examination of a biopsied tissue sample remains the definitive method for diagnosing melanoma. The tissue is processed, thinly sectioned, and stained with hematoxylin and eosin (H&E) for microscopic evaluation by a pathologist. This analysis confirms the diagnosis based on key cellular features, such as atypical melanocyte proliferation, invasion depth (Breslow depth), ulceration, and mitotic rate, which are essential for staging and prognosis.
Immunohistochemistry (IHC): Refining the Diagnosis
IHC is a powerful ancillary technique that uses antibodies to detect specific protein markers in the tissue. When melanoma diagnosis is challenging based on H&E morphology alone, IHC markers provide objective data. Stains for markers like SOX10, S100, Melan-A, and HMB-45 confirm the melanocytic origin of the cells, while Ki-67 helps assess proliferative activity, aiding in distinguishing melanoma from benign mimics or other cancer types.
Molecular testing has revolutionized melanoma diagnostics by providing critical genetic insights that extend beyond traditional pathology. It enables precise confirmation of diagnosis in challenging cases, delivers valuable prognostic information, and most importantly, identifies actionable therapeutic targets for personalized treatment, fundamentally improving clinical decision-making and patient outcomes.
Key Genomic Alterations in Melanoma
Melanoma is driven by distinct, therapeutically relevant genomic alterations that guide diagnosis and treatment. The most critical of these include:
Diagnostic and Prognostic Molecular Tests
Advanced molecular techniques are essential for detecting genomic alterations in melanoma, enabling precise diagnosis and guiding treatment decisions. The primary platforms include:
Alta DiagnoTech provides a comprehensive portfolio of in vitro diagnostic (IVD) solutions to support the accurate diagnosis, prognostic stratification, and treatment selection for melanoma. Our robust and validated assays empower pathology laboratories to deliver precise molecular profiling, enabling personalized patient management from initial detection to therapeutic decision-making. If you have related needs, please feel free to contact us for more information or product support.
| Product Name | Technology | Application |
| BRAF V600E/K Mutation Detection Kit | Real-Time PCR | Detection of specific BRAF mutations for targeted therapy selection |
| NRAS Mutation Detection Kit | PCR & Sanger Sequencing | Identification of NRAS Q61/R13 mutations for prognosis and clinical trial eligibility |
| c-KIT Mutation Detection Kit | PCR & Sanger Sequencing | Profiling of c-KIT mutations in acral and mucosal melanoma subtypes |
| Melanoma IHC Marker Panel (SOX10, HMB-45, Melan-A) | Immunohistochemistry (IHC) | Aid in the differential diagnosis of melanocytic lesions |
| Comprehensive Solid Tumor NGS Panel | Next-Generation Sequencing (NGS) | Simultaneous detection of mutations across 50+ genes including BRAF, NRAS, c-KIT, and TMB analysis |
| PD-L1 IHC Assay (Clone 22C3) | Immunohistochemistry (IHC) | Assessment of PD-L1 expression levels to guide immunotherapy |
Reference
This article is for research use only. Do not use in any diagnostic or therapeutic application.
|
There is no product in your cart. |