- Home
- Resource
- Disease Diagnosis
- Genetic Diseases
- Duchenne Muscular Dystrophy (DMD) Diagnosis: From CK Screening to Genetic Confirmation
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Duchenne muscular dystrophy (DMD) is a severe, progressive genetic disorder caused by mutations in the DMD gene, leading to muscle degeneration and functional decline. Early and accurate diagnosis is critical to initiate timely interventions and improve patient outcomes. This resource provides a comprehensive overview of the step-by-step diagnostic pathway, from initial CK screening to confirmatory genetic testing and specialized muscle biopsy.
Duchenne muscular dystrophy (DMD) is a severe, progressive neuromuscular disorder caused by mutations in the DMD gene, which encodes the dystrophin protein. As an X-linked recessive condition, DMD primarily affects males, with an incidence of approximately 1 in 3,500–5,000 live male births. The absence of functional dystrophin leads to muscle fiber degeneration, resulting in progressive weakness, loss of ambulation, and eventual cardiac and respiratory complications.
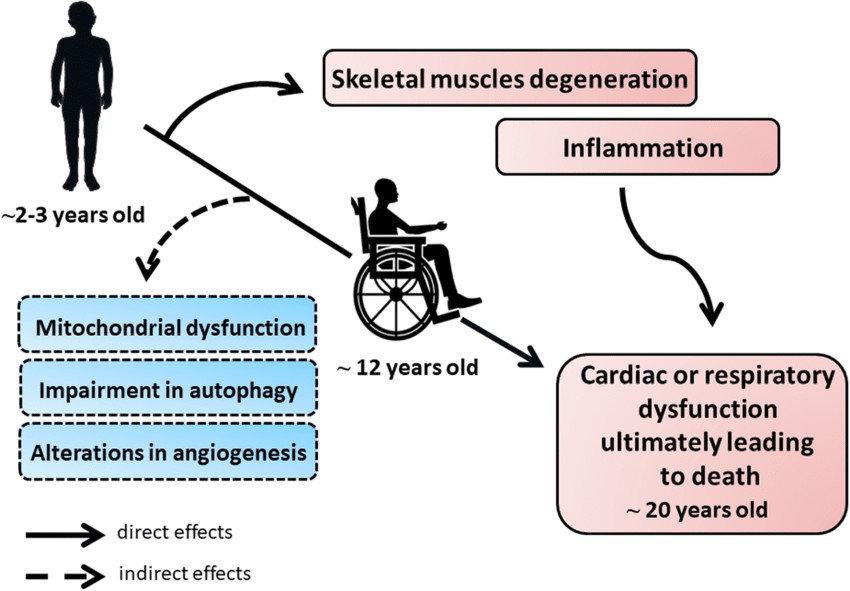 Fig.1 The pathology of Duchenne muscular dystrophy (DMD). (Podkalicka P, et al., 2019)
Fig.1 The pathology of Duchenne muscular dystrophy (DMD). (Podkalicka P, et al., 2019)
Early diagnosis is critical to initiate timely interventions, such as corticosteroid therapy and multidisciplinary care, which can slow disease progression and improve quality of life. The diagnostic pathway typically begins with clinical suspicion (e.g., delayed motor milestones, elevated serum creatine kinase (CK) levels), followed by genetic testing (e.g., multiplex ligation-dependent probe amplification [MLPA], next-generation sequencing [NGS]) to identify mutations. In cases where genetic results are inconclusive, muscle biopsy with dystrophin immunohistochemistry provides definitive confirmation.
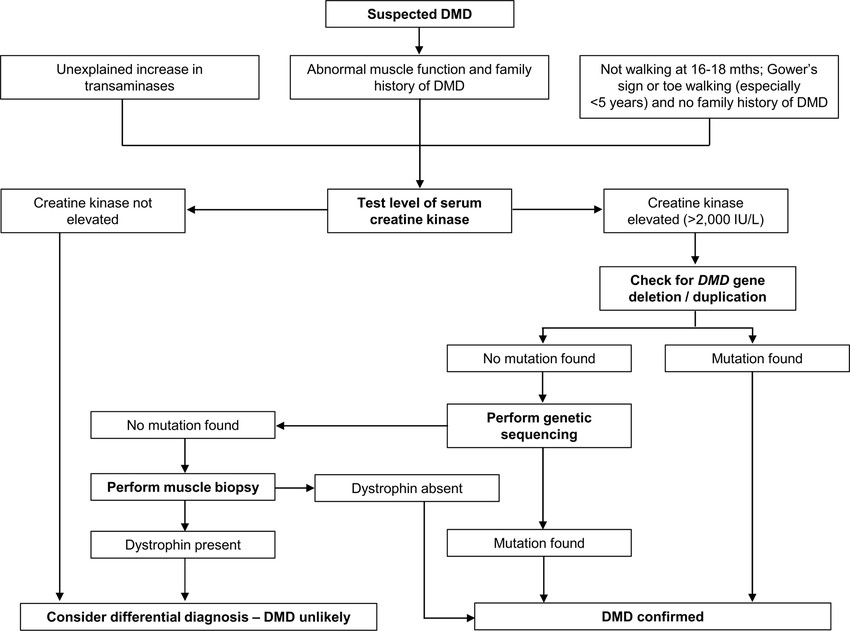 Fig.2 Diagnostic algorithm for Duchenne muscular dystrophy (DMD). (Mercuri E, et al., 2023)
Fig.2 Diagnostic algorithm for Duchenne muscular dystrophy (DMD). (Mercuri E, et al., 2023)
Creatine kinase (CK) is a critical initial biomarker in the diagnostic workup of Duchenne muscular dystrophy (DMD). As an enzyme released into the bloodstream during muscle damage, elevated CK levels often serve as the first laboratory indication of DMD, prompting further investigation.
Interpretation of CK Levels
In DMD patients, CK levels are typically elevated 10-100 times above normal ranges (often >5,000 U/L, with some cases exceeding 20,000 U/L). This dramatic elevation can be detected as early as infancy, making CK testing particularly useful for early suspicion of DMD. Persistent high CK levels in a male child with motor delays strongly warrants genetic testing for confirmation.
Limitations of CK Testing
Genetic testing represents the gold standard for confirming Duchenne muscular dystrophy (DMD), as it identifies pathogenic mutations in the DMD gene. This step is critical not only for definitive diagnosis but also for guiding therapeutic decisions, genetic counseling, and family planning.
MLPA is the primary method for detecting large deletions or duplications in the DMD gene, which account for approximately 70% of cases. This targeted technique analyzes copy number variations across all 79 exons, providing a cost-effective and efficient way to identify the most common genetic abnormalities in DMD. Its high throughput and reliability make it the first-line genetic test in most diagnostic workflows.

For the remaining 30% of cases not resolved by MLPA, typically caused by point mutations or small indels, NGS offers comprehensive sequencing of the entire DMD gene. This advanced method can detect subtle variants, including nonsense, splice-site, and deep intronic mutations, ensuring a high diagnostic yield. NGS is particularly valuable for atypical presentations or when MLPA results are negative.
Genetic testing identifies most DMD mutations but faces challenges like complex variants or false negatives, requiring muscle biopsy when results are inconclusive despite clinical suspicion, atypical presentations, or need to quantify dystrophin for differential diagnosis.
When genetic testing fails to confirm a Duchenne muscular dystrophy (DMD) diagnosis despite strong clinical suspicion, muscle biopsy serves as a definitive diagnostic tool. This procedure analyzes muscle tissue for dystrophin expression, providing critical insights when genetic results are inconclusive or when differentiating between Duchenne and Becker muscular dystrophy is necessary.
A confirmed Duchenne muscular dystrophy (DMD) diagnosis triggers immediate, coordinated interventions: initiating corticosteroid therapy to preserve muscle function, implementing cardiac/respiratory surveillance protocols, and referring to neuromuscular specialists for comprehensive care. Genetic results also guide targeted therapies and inform family counseling regarding carrier testing, reproductive options, and clinical trial enrollment. Early multidisciplinary action optimizes outcomes and quality of life.
As a trusted provider of in vitro diagnostic (IVD) solutions, Alta DiagnoTech offers a full range of high-quality Duchenne muscular dystrophy (DMD) testing products, including CK assay kits, MLPA kits, and NGS kits to ensure accurate and efficient diagnostics at every stage. If you have related needs, please feel free to contact us for more information or product support.
References
| Cat.No | Product Name | Price |
|---|---|---|
| KP-HYW-0001 | Human SMN1/DMD/FMR1 Mutation Detection Kit | Add To Cart |
| NG-QCY-0001 | NGS Kit | Add To Cart |
| NG-QCY-0002 | Library Preparation NGS Kit | Add To Cart |
| GW-00199 | Single cell 3' RNA sequencing kit | Add To Cart |
| NG-QCY-0004 | Small RNA Index Primer Kit | Add To Cart |
| NG-QCY-0003 | Single Cell NGS Kit | Add To Cart |
| NG-QCY-0005 | DNA Fragmentation Kit by NGS | Add To Cart |
This article is for research use only. Do not use in any diagnostic or therapeutic application.
|
There is no product in your cart. |