- Home
- Resource
- Disease Diagnosis
- Cancers
- Diagnostic Pathway for Thyroid Cancer: From Screening to Precision Management
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Thyroid cancer is a disease in which malignant cells form in the tissues of the thyroid gland. This resource provides a comprehensive guide to its diagnostic pathway, detailing the essential steps from initial clinical suspicion and imaging through pathological confirmation, and highlighting the critical role of laboratory biomarkers for precise diagnosis and long-term management.
Thyroid cancer originates in the tissues of the thyroid gland, a butterfly-shaped organ at the base of the neck responsible for regulating metabolism. While thyroid nodules are exceedingly common, the vast majority are benign; only a small percentage are malignant. The disease encompasses several main types, with differentiated thyroid cancers like papillary and follicular carcinoma being the most prevalent and typically having an excellent prognosis. Diagnosis often begins with the detection of a nodule, either by palpation or incidentally through imaging, triggering a systematic diagnostic pathway that utilizes ultrasound, biopsy, and key biomarker tests to achieve a precise diagnosis and guide effective, individualized management.
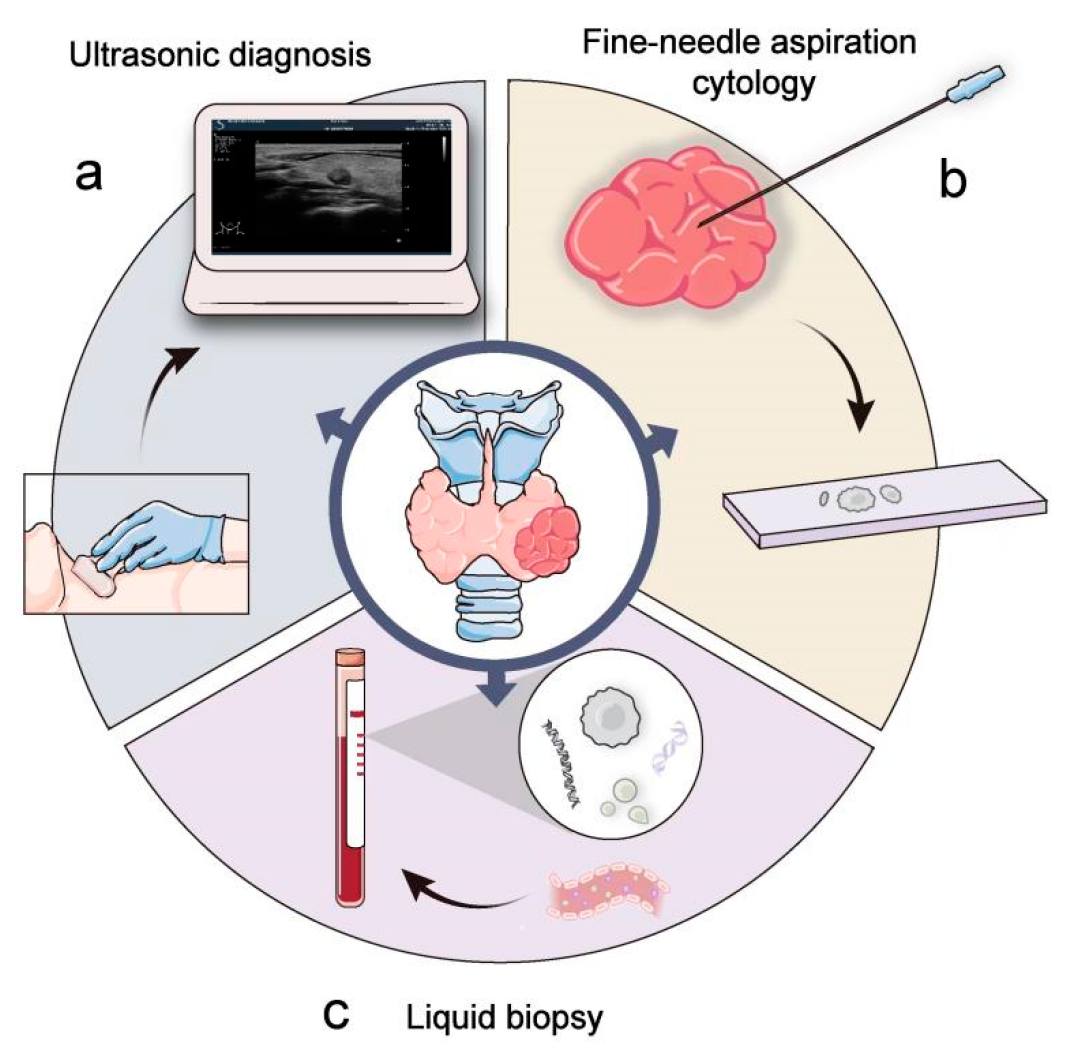 Fig.1 Clinical examination and diagnosis of thyroid cancer. (Wang W, et al., 2023)
Fig.1 Clinical examination and diagnosis of thyroid cancer. (Wang W, et al., 2023)
Thyroid imaging is the cornerstone of the initial assessment following the clinical suspicion of thyroid cancer, serving to visualize the gland's structure, identify suspicious nodules, and guide further diagnostic steps. It provides the essential anatomical roadmap that determines the need for and approach to subsequent procedures like biopsy. The evaluation primarily relies on ultrasound for its detailed resolution of the thyroid itself, while supplementary modalities are reserved for assessing the broader context of disease spread.

As the first-line imaging tool, thyroid ultrasound provides high-resolution visualization of the gland. It is essential for characterizing nodules—assessing their size, composition, and echogenicity—and for identifying suspicious features like microcalcifications. This detailed evaluation allows for risk stratification using systems like TI-RADS, which directly determines the need for fine-needle aspiration (FNA) biopsy.

These advanced modalities are reserved for staging, not initial diagnosis. CT and MRI are pivotal for evaluating large or invasive tumors, assessing lymph node involvement beyond the thyroid bed, and detecting distant metastases. PET-CT is primarily used for locating recurrent or metastatic disease, especially in cases where it is not iodine-avid.
Pathological confirmation is the definitive step in diagnosing thyroid cancer, transforming imaging findings into a conclusive diagnosis. This process provides the cellular evidence required to confirm malignancy and often, its specific type.
Ultrasound-Guided Fine-Needle Aspiration (FNA) Biopsy
This minimally invasive procedure is the gold standard for obtaining thyroid cells for diagnosis. Using ultrasound for real-time guidance ensures accurate sampling of the target nodule, improving both the safety and diagnostic yield of the biopsy.

Cytopathology Reporting
The aspirated cells are analyzed under a microscope by a pathologist. The findings are typically reported using the Bethesda System, which categorizes results into standardized diagnostic classes. This classification directly guides clinical management, from recommending surveillance to surgery.
Laboratory medicine is fundamental to the precision management of thyroid cancer, moving beyond anatomical imaging to provide critical molecular insights. Specific biomarkers deliver essential information for diagnosis, risk stratification, and post-treatment surveillance, enabling highly individualized patient care.
Pre-Operative & Diagnostic Biomarkers
These biomarker tests are crucial for preoperative evaluation of thyroid nodules. They provide critical molecular information that complements imaging findings, aiding in diagnosis, determining the specific cancer type, and assessing risk. Key biomarkers include:
Post-Operative & Monitoring Biomarkers
Following treatment, these biomarkers become the cornerstone of long-term patient management. They are used to detect the presence of residual disease, monitor for recurrence, and assess the effectiveness of therapy. The most important markers are:
Alta DiagnoTech provides a comprehensive suite of high-precision in vitro diagnostic (IVD) solutions for thyroid cancer, supporting the entire patient journey from initial diagnosis to long-term surveillance. Our robust assays deliver the critical data clinicians need for accurate risk stratification, definitive diagnosis, and precise monitoring, enabling personalized patient management. If you have related needs, please feel free to contact us for more information or product support.
| Product Name | Technology | Application |
| Calcitonin Detection Kit | Chemiluminescent Immunoassay (CLIA) | Quantitative measurement of serum calcitonin for the diagnosis and monitoring of medullary thyroid carcinoma (MTC). |
| Thyroglobulin (Tg) Detection Kit | Chemiluminescent Immunoassay (CLIA) | Highly sensitive quantification of serum Tg for monitoring patients with differentiated thyroid cancer (DTC). |
| Anti-Thyroglobulin Antibody (TgAb) Detection Kit | Chemiluminescent Immunoassay (CLIA) | Detection of thyroglobulin antibodies to ensure the accurate interpretation of Tg results. |
| Carcinoembryonic Antigen (CEA) Detection Kit | Chemiluminescent Immunoassay (CLIA) | Precise measurement of CEA as an adjunctive tool in the management of medullary thyroid carcinoma. |
| Thyroid Function Panel | Chemiluminescent Immunoassay (CLIA) | Simultaneous testing of key thyroid function indicators, including TSH, for baseline functional assessment. |
Reference
This article is for research use only. Do not use in any diagnostic or therapeutic application.
|
There is no product in your cart. |