- Home
- Resource
- Disease Diagnosis
- Metabolic Diseases
- Diagnosing the Porphyrias: A Stepwise Laboratory Approach
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
The accurate diagnosis of porphyrias demands a systematic laboratory strategy to navigate their complex and often overlapping clinical presentations. This resource outlines a definitive stepwise approach, progressing from initial biomarker screening to comprehensive differentiation and final confirmation. The following sections will detail this critical pathway, including first-line biochemical tests, second-line porphyrin profiling, and definitive enzymatic or molecular analysis, to provide a clear roadmap for precise diagnosis and effective patient management.
The porphyrias are a group of rare metabolic disorders resulting from specific enzyme deficiencies in the heme biosynthesis pathway, leading to the abnormal accumulation of porphyrins and their precursors. These conditions are clinically categorized into two primary groups based on the dominant symptomology: the acute porphyrias, which present with potentially life-threatening, episodic neurovisceral attacks characterized by severe abdominal pain, autonomic dysfunction, and neuropsychiatric symptoms; and the cutaneous porphyrias, where the predominant manifestation is photosensitivity, causing skin fragility, blistering, and scarring upon sun exposure. This fundamental clinical distinction is the critical first step in guiding an effective and targeted diagnostic laboratory workup.
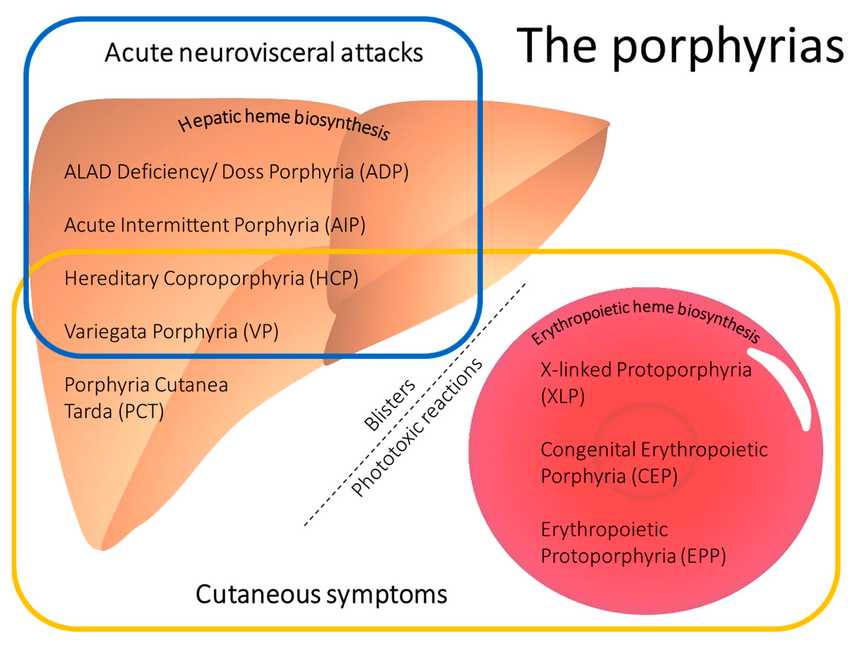 Fig.1 Main clinical characteristics of the porphyrias. (Belosevic, et al., 2023)
Fig.1 Main clinical characteristics of the porphyrias. (Belosevic, et al., 2023)
The cornerstone of porphyria diagnosis lies in first-line biochemical screening, which utilizes specific, high-yield tests to confirm clinical suspicion and guide further investigation. This initial step is crucial for rapidly identifying or ruling out active disease, with the test selection being directly determined by the patient's predominant symptoms—either acute neurovisceral or cutaneous. The following approaches target the key biomarkers that are pathognomonic for these presentations.

When an acute porphyria attack is suspected, the single most critical and urgent test is the quantitative measurement of porphobilinogen (PBG) in a random urine sample. A significant elevation in urinary PBG is the biochemical hallmark of an acute attack, confirming the presence of one of the acute porphyrias (AIP, VP, or HCP). It is imperative that the sample is collected during the acute episode, as PBG levels may normalize during remission, and the result must be quantitative to assess the degree of elevation accurately. Concurrent measurement of urinary delta-aminolevulinic acid (ALA) is also typically performed.
For patients presenting with skin photosensitivity, the most sensitive and specific first-line screening test is plasma porphyrin fluorescence scanning. This method detects the characteristic fluorescence emission peak of circulating porphyrins and is highly effective for screening all cutaneous porphyrias. A positive result confirms a diagnosis of porphyria, and the specific fluorescence peak can often help differentiate between the various types. Alternatively, a quantitative test for total plasma porphyrins can serve as an initial screen, though it lacks the differentiating power of the fluorescence scan.
When first-line screening returns a positive or equivocal result, the diagnostic process advances to second-line analysis through comprehensive porphyrin profiling, a crucial step that moves beyond simple detection to precise differentiation of the specific porphyria type. This involves the quantitative chromatographic separation and measurement of the unique patterns and ratios of porphyrins and their precursors across different biological matrices—primarily urine, feces, and blood. The distinct porphyrin "fingerprint" identified in each sample type is diagnostically definitive, allowing clinicians to distinguish between acute porphyrias with similar PBG elevations and to accurately classify the various cutaneous porphyrias based on their characteristic accumulation and excretion profiles.
Following a positive biochemical profile, the diagnostic pathway proceeds to definitive confirmation, which aims to identify the precise functional or genetic defect underlying the disease. This crucial step moves from demonstrating the biochemical abnormality to directly pinpointing its root cause, providing irrefutable evidence for the diagnosis and creating a foundation for family studies. The two primary methods for achieving this confirmation are enzyme activity assays and molecular genetic testing.

Enzyme Activity Assays
Enzyme activity assays provide functional confirmation by directly measuring the catalytic function of the specific enzyme deficient in a particular porphyria. For example, this involves quantifying the activity of enzymes such as porphobilinogen deaminase (for AIP) or uroporphyrinogen decarboxylase (for PCT) in erythrocytes or other relevant cell types. A significantly reduced enzyme activity provides definitive biological proof of the metabolic defect and is particularly valuable for family screening once the proband's specific deficiency is known.
Molecular Genetic Testing
Molecular genetic testing, primarily through DNA sequencing, offers the ultimate level of diagnostic certainty by identifying the specific pathogenic gene variants responsible for the enzyme deficiency. This method detects mutations in genes such as HMBS, UROD, CPOX, or PPOX, delivering a definitive etiological diagnosis. The identification of causative mutations is indispensable for enabling precise carrier detection, predictive testing for at-risk asymptomatic relatives, and informed genetic counseling.
Navigating the complex diagnostic pathway for porphyrias requires precise and reliable laboratory tools. Alta DiagnoTech provides a comprehensive suite of in vitro diagnostic (IVD) solutions that support the entire stepwise diagnostic approach—from initial symptomatic screening to definitive confirmation—enabling clinical laboratories to deliver accurate and timely diagnoses for these challenging disorders. If you have related needs, please feel free to contact us for more information or product support.
| Product Category | Product Name | Application |
| First-Line Screening | Urinary PBG & ALA Quantitative Assay Kit | First-line, urgent testing for suspected acute porphyria attacks. |
| Plasma Porphyrin Fluorescence Screening Kit | Initial screening for all cutaneous porphyrias. | |
| Second-Line Profiling | Comprehensive Porphyrin Profile Kit (Urine, Feces, Plasma) | Differentiation and subtyping of porphyrias after a positive screen. |
| Definitive Confirmation | Porphyria Enzyme Activity Assay Panel | Functional confirmation of specific enzymatic deficiencies (e.g., PBGD, UROD). |
| Porphyria Genetic Testing Panel | Molecular confirmation and carrier testing. |
Reference
This article is for research use only. Do not use in any diagnostic or therapeutic application.
|
There is no product in your cart. |