- Home
- Resource
- Disease Diagnosis
- Metabolic Diseases
- Diagnosing Organic Acidemias (OAs): A Guide to Integrated Laboratory Strategies
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
The diagnosis of organic acidemias (OAs) has evolved into a sophisticated, multi-stage process crucial for initiating timely and life-altering patient management. This resource page details the integrated laboratory strategy that forms the modern standard of care, moving from initial biomarker screening to definitive molecular confirmation.
Organic acidemias (OAs) are a group of rare, inherited metabolic disorders characterized by defects in the enzymatic pathways that break down amino acids, fats, and other organic compounds. This results in the toxic accumulation of specific organic acids and their derivatives (such as acylcarnitines) within the body's cells and fluids. The accumulation disrupts fundamental biochemical processes, often leading to severe, life-threatening symptoms in newborns and infants, including metabolic acidosis, lethargy, vomiting, and developmental delay. Because their presentation is often non-specific and acute, a rapid and accurate multi-tiered laboratory diagnosis is critical for initiating timely treatment and managing the long-term health of affected individuals.
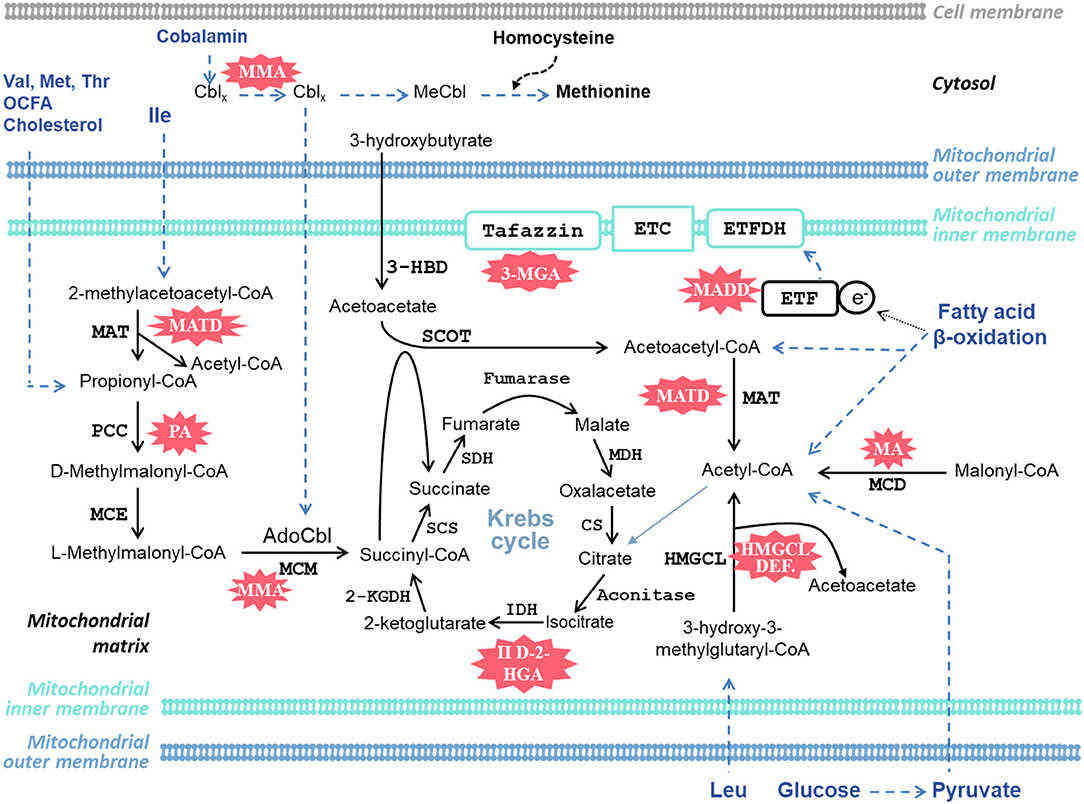 Fig.1 Schematic representation of cardiac-specific metabolic pathways involved in organic acidemias (OAs). (Park, et al., 2020)
Fig.1 Schematic representation of cardiac-specific metabolic pathways involved in organic acidemias (OAs). (Park, et al., 2020)

The critical first line of defense in identifying organic acidemias (OAs) is newborn screening, which primarily utilizes tandem mass spectrometry (MS/MS) to analyze dried blood spots. This high-throughput technology profiles acylcarnitines, which are key biomarker derivatives of organic acids. By detecting characteristic elevations in specific acylcarnitine species, such as propionylcarnitine (C3) for propionic acidemia, this non-invasive screening provides the earliest possible indication of a potential underlying OA, triggering the urgent confirmatory testing necessary for a definitive diagnosis and timely intervention.
Following an abnormal newborn screening result, the definitive biochemical step for diagnosing most organic acidemias (OAs) is the urinary organic acid analysis by gas chromatography-mass spectrometry (GC-MS). This technique is considered the gold standard because it moves beyond indirect biomarkers to provide a comprehensive "metabolic fingerprint," directly identifying and quantifying the specific pathological organic acids that accumulate due to the enzymatic block. It is this specific pattern of metabolites that allows for a conclusive biochemical diagnosis.
Urinary Organic Acid Analysis by GC-MS
Gas chromatography-mass spectrometry (GC-MS) is an analytical technique that separates complex mixtures (gas chromatography) and then identifies each component with high specificity (mass spectrometry). In the context of OAs, it is used to profile the unique spectrum of organic acids in a urine sample. For instance, it can definitively identify elevated levels of methylmalonic acid for methylmalonic acidemia, isovalerylglycine for isovaleric acidemia, or a combination of 3-hydroxy-3-methylglutaric acid and 3-methylglutaconic acid for 3-hydroxy-3-methylglutaryl-CoA lyase deficiency. This level of specificity is crucial for distinguishing between different OAs that may present with similar clinical features or initial screening results.
Timing and Interpretation
The timing of sample collection is critical for an accurate analysis. The highest levels of diagnostic metabolites are excreted during an acute metabolic decompensation. A sample collected when a patient is clinically stable may be normal or show only subtle abnormalities, leading to a false negative. Furthermore, interpreting a GC-MS profile requires significant expertise, as secondary metabolite elevations can be influenced by factors like diet, intestinal flora, antibiotics, and overall clinical status. Therefore, correlation with the patient's condition and other laboratory findings is essential for a correct diagnosis.
Once biochemical profiling strongly suggests a specific organic acidemia (OA), the diagnostic process moves to a confirmatory tier. This crucial step employs specialized assays to move from inferring the metabolic block to directly demonstrating its fundamental cause, either by measuring the deficient enzymatic function itself or by identifying the definitive genetic mutation responsible.
Enzyme activity assays provide direct functional confirmation of the diagnosis by quantitatively measuring the activity of a specific enzyme, typically in cultured fibroblasts or lymphocytes. For example, this assay can confirm a diagnosis of propionic acidemia by demonstrating deficient activity of propionyl-CoA carboxylase in a patient's cells, providing definitive biological evidence of the metabolic defect.

Molecular genetic testing, often using DNA sequencing techniques like next-generation sequencing (NGS) panels, delivers the ultimate etiological confirmation by identifying the pathogenic variants in the genes associated with organic acidemias. Identifying biallelic mutations in a gene such as MUT for methylmalonic acidemia not only confirms the diagnosis with certainty but also enables precise carrier testing for family members and informs genetic counseling.
Diagnosis of organic acidemias (OAs) requires a seamless, multi-stage laboratory strategy. Alta DiagnoTech's in vitro diagnostic (IVD) solutions are designed to support the entire diagnostic process, simplifying the path to accurate diagnosis by providing laboratories with the critical tools needed for high-throughput newborn screening, precise biochemical confirmation, and ultimately genetic validation. If you have related needs, please feel free to contact us for more information or product support.
| Product Category | Product Name | Application |
| Newborn Screening | Acylcarnitine Profile Screening Kit (DBS) | First-tier newborn screening for OAs using dried blood spots. |
| Biochemical Confirmation | Urinary Organic Acid Analysis Kit (GC-MS) | Definitive biochemical confirmation and differentiation of specific OAs. |
| Enzymatic Confirmation | Enzyme Activity Assay Panel for OAs | Functional confirmation of specific enzymatic deficiencies (e.g., Propionyl-CoA Carboxylase, Methylmalonyl-CoA Mutase). |
| Genetic Confirmation | Organic Acidemias Genetic Testing Panel | Molecular genetic confirmation and carrier testing. |
Reference
This article is for research use only. Do not use in any diagnostic or therapeutic application.
|
There is no product in your cart. |