- Home
- Resource
- Disease Diagnosis
- Genetic Diseases
- Diagnosing Hereditary Hemochromatosis: From Serum Biomarkers to Genetic Confirmation
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Hereditary hemochromatosis is a common genetic disorder of iron overload that, if undiagnosed, can lead to severe damage to the liver, heart, and other organs. This resource details the essential diagnostic pathway, providing a clear guide from initial clinical suspicion through the foundational step of serum iron biomarker testing (transferrin saturation and ferritin), to definitive genetic confirmation with HFE gene analysis.
Hereditary hemochromatosis is a common genetic disorder characterized by excessive intestinal absorption of dietary iron, leading to progressive iron overload in the body's tissues and organs. It is most often caused by homozygous mutations in the HFE gene (particularly C282Y). The accumulated iron can cause oxidative damage over time, resulting in complications such as liver cirrhosis, diabetes, cardiomyopathy, and arthritis. Diagnosis follows a sequential pathway, beginning with screening using serum iron biomarkers (elevated transferrin saturation and ferritin) and confirmed by genetic testing for HFE mutations, which is essential for differentiating it from secondary causes of iron overload.
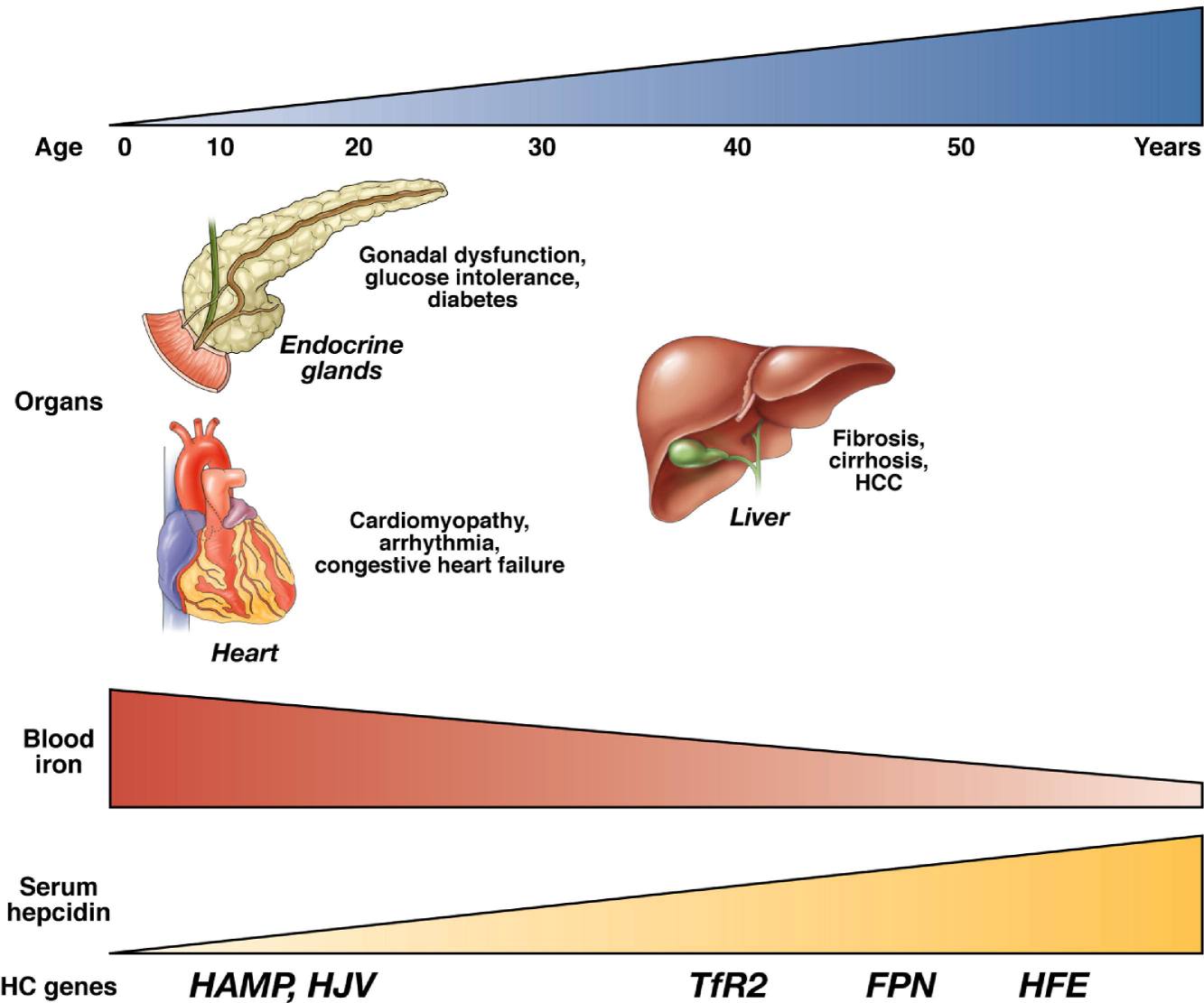 Fig.1 The common genetic basis and phenotypic continuum of hemochromatosis. (Pietrangelo, Antonello., 2010)
Fig.1 The common genetic basis and phenotypic continuum of hemochromatosis. (Pietrangelo, Antonello., 2010)

Initial testing for hereditary hemochromatosis should be prompted by non-specific clinical symptoms, incidental laboratory findings, or a relevant family history. Key clinical indicators include unexplained chronic fatigue, joint pain (especially in the knuckles), abdominal discomfort, loss of libido, or skin discoloration (bronze or gray). Laboratory triggers often involve elevated liver enzymes (AST, ALT) on routine blood work. A family history of hemochromatosis in a first-degree relative is one of the strongest indications for testing. Additionally, the presence of associated conditions such as unexplained liver disease, cardiomyopathy, or diabetes should raise clinical suspicion and warrant an initial iron panel.
The measurement of serum iron biomarkers is the critical first laboratory step in evaluating suspected hemochromatosis, serving to screen for and quantify the presence of systemic iron overload. This initial "iron panel" provides objective, biochemical evidence that guides all subsequent diagnostic decisions.
Transferrin Saturation (TSAT)
Transferrin saturation (TSAT) is calculated as (serum iron / total iron-binding capacity) x 100%. It is the most sensitive initial screening test for hemochromatosis, reflecting the percentage of iron-binding sites on transferrin that are occupied. A persistently elevated TSAT (typically >45-55%) indicates increased iron absorption and is often the earliest biochemical abnormality detected, even before ferritin rises or symptoms appear.
Serum Ferritin
Serum ferritin directly measures the body's total iron storage levels. It is the key biomarker for assessing the magnitude of iron overload, with levels roughly correlating with total body iron stores. Importantly, serum ferritin is also an acute-phase reactant, meaning levels can be falsely elevated due to inflammation, infection, liver cell injury, or malignancy, which must be considered during interpretation to avoid misdiagnosis.
Genetic testing provides the definitive confirmation for hereditary hemochromatosis, moving beyond biochemical indicators to identify the underlying genetic cause. It is primarily used to detect pathogenic mutations in the HFE gene, which distinguish the hereditary form from secondary causes of iron overload and enable precise family screening.

The core of genetic testing focuses on analyzing the HFE gene. The vast majority of hereditary hemochromatosis cases in populations of Northern European descent are linked to two specific mutations: the p.Cys282Tyr (C282Y) variant and the p.His63Asp (H63D) variant. Diagnosis is typically confirmed by identifying a homozygous C282Y/C282Y genotype or a compound heterozygous C282Y/H63D genotype.

A positive genetic test confirms a genetic predisposition to iron overload, but it does not predict the severity of clinical disease. This is due to incomplete penetrance; not all individuals with the high-risk genotypes (especially C282Y homozygotes) will develop significant iron overload or symptoms. The phenotypic expression is influenced by other genetic, environmental, and lifestyle factors, such as alcohol consumption and blood loss.

Genetic testing plays a crucial role in differential diagnosis by definitively ruling in hereditary hemochromatosis and ruling out secondary iron overload (e.g., from anemia or chronic liver disease). Once a pathogenic mutation is identified in an index patient, it enables highly efficient and targeted cascade screening of first-degree relatives, who can be tested specifically for the known familial mutation to determine their own risk status early.
To address the critical need for early detection and accurate differentiation of iron overload disorders, Alta DiagnoTech provides comprehensive in vitro diagnostic (IVD) solutions for hereditary hemochromatosis. Our portfolio supports the complete diagnostic cascade, from the initial biochemical screening for iron overload to the definitive molecular confirmation of the genetic cause, empowering laboratories to deliver precise results that guide clinical management and family screening. If you have related needs, please feel free to contact us for more information or product support.
| Product Name | Technology | Application |
| Serum Iron & TIBC (Total Iron-Binding Capacity) Assay Kit | Colorimetric / Enzymatic Assay | Quantitative measurement of serum iron and TIBC for the calculation of Transferrin Saturation (TSAT), the primary screening biomarker for iron overload. |
| Ferritin Immunoassay Kit | Chemiluminescent Microparticle Immunoassay (CMIA) / Electrochemiluminescence (ECLIA) | Precise quantitative measurement of serum ferritin levels to assess total body iron stores and monitor the magnitude of iron overload and response to therapy. |
| HFE Genotyping Kit (C282Y, H63D) | Real-Time PCR with Fluorescent Probe Detection | Targeted, high-throughput detection of the two most common pathogenic variants (p.Cys282Tyr and p.His63Asp) in the HFE gene for definitive diagnosis and genetic counseling. |
| Extended Hereditary Iron Overload Genetic Panel | Next-Generation Sequencing (NGS) Panel | Comprehensive analysis of genes associated with hereditary iron overload (including HFE, HJV, HAMP, TFR2, SLC40A1) for diagnosing atypical or juvenile-onset cases and differential diagnosis. |
| Liver Function Test (LFT) Panel | Integrated Multi-Analyte Profiling | Simultaneous measurement of key liver enzymes (ALT, AST, ALP) and proteins (Albumin, Total Protein) to assess potential liver damage resulting from iron deposition. |
Reference
This article is for research use only. Do not use in any diagnostic or therapeutic application.
|
There is no product in your cart. |