- Home
- Resource
- Disease Diagnosis
- Autoimmune Diseases
- Diagnosing Graves' Disease: Integrating Clinical Assessment with Advanced Laboratory Analysis
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Graves' disease is an autoimmune disorder and the leading cause of hyperthyroidism. This resource details its integrated diagnostic approach, beginning with recognizing clinical signs and proceeding through a definitive laboratory pathway to confirm the diagnosis and rule out other conditions. It also covers the role of advanced diagnostics in management and highlights relevant IVD solutions.
Graves' disease is an autoimmune disorder and the most common cause of hyperthyroidism, characterized by the production of autoantibodies that mistakenly stimulate the thyroid-stimulating hormone (TSH) receptor. This leads to uncontrolled overproduction of thyroid hormones (T4 and T3), resulting in a state of thyrotoxicosis. The classic clinical presentation includes symptoms such as weight loss despite increased appetite, heat intolerance, palpitations, anxiety, and tremors, alongside distinctive physical signs like diffuse goiter, thyroid eye disease (ophthalmopathy), and occasionally pretibial myxedema. Diagnosis relies on the integration of clinical assessment with targeted laboratory analysis, typically beginning with a suppressed TSH level and elevated free thyroid hormones, and is confirmed by the detection of TSH receptor antibodies (TRAb).
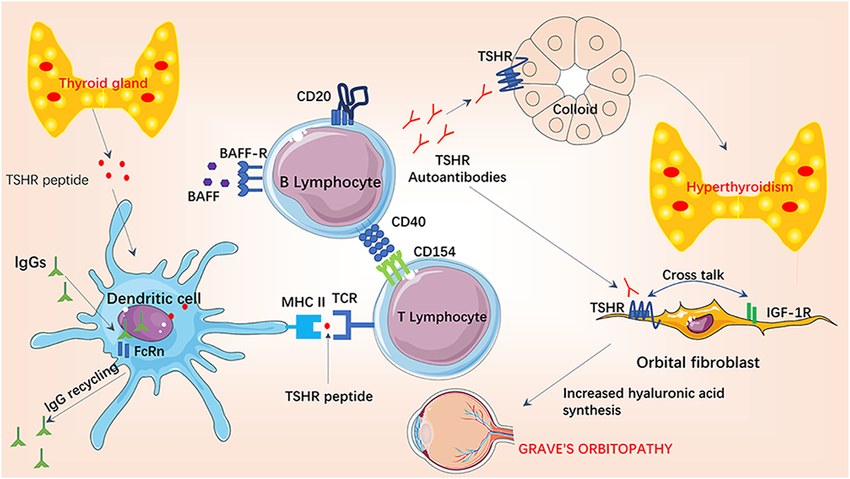 Fig.1 Pathogenesis of Graves' hyperthyroidism and Graves' orbitopathy. (He, Qiongyao, et al., 2022)
Fig.1 Pathogenesis of Graves' hyperthyroidism and Graves' orbitopathy. (He, Qiongyao, et al., 2022)
Clinical suspicion of Graves' disease arises from recognizing its characteristic yet variable clinical presentation. Key indicators include classic hyperthyroid symptoms (such as unexplained weight loss, heat intolerance, palpitations, anxiety, and tremor) combined with distinctive physical signs like a diffusely enlarged, often vascular goiter (which may present with a palpable thrill or audible bruit) and the unique extrathyroidal manifestations of ophthalmopathy (e.g., lid retraction, proptosis) or, rarely, pretibial myxedema. However, presentation can be subtle or atypical, especially in the elderly, who may present with "apathetic hyperthyroidism" dominated by fatigue, depression, and atrial fibrillation, or when isolated symptoms like unexplained weight loss or tachycardia are the primary concern.
Purpose of Clinical Assessment
The purpose of this initial clinical assessment is twofold: first, to identify the pattern of signs and symptoms that warrants further biochemical investigation to confirm thyrotoxicosis, and second, to begin the critical process of differential diagnosis by gathering clues that help distinguish Graves' disease from other causes of hyperthyroidism, such as thyroiditis or toxic nodular goiter, thereby determining the most appropriate and efficient laboratory testing pathway.
Following clinical suspicion, a structured laboratory diagnostic pathway is essential to objectively confirm the presence of thyrotoxicosis and, most critically, to pinpoint Graves' disease as its specific autoimmune cause. This tiered approach begins with broad screening tests and proceeds to targeted, disease-specific assays, ensuring an accurate and efficient diagnosis while systematically ruling out other conditions.
Initial Testing – Confirming Thyrotoxicosis
The primary goal of initial testing is to biochemically verify the state of hyperthyroidism. This is most effectively and efficiently achieved by measuring a sensitive thyroid-stimulating hormone (TSH) assay. A significantly suppressed or undetectable TSH level is the hallmark of primary thyrotoxicosis. To confirm the diagnosis and assess its severity, this is followed by measuring free thyroxine (FT4) and/or free triiodothyronine (FT3). An elevated FT4 and/or FT3 confirms the diagnosis. Notably, measuring FT3 is crucial for identifying "T3 toxicosis," a condition where only T3 is elevated, which can occur in early or mild Graves' disease.

Differential Diagnosis – Identifying the Etiology
Once thyrotoxicosis is confirmed, the next critical step is to determine its underlying cause. The definitive test for Graves' disease is the measurement of TSH receptor antibodies (TRAb). A positive TRAb test is specific for Graves' disease, as it directly detects the pathogenic autoantibodies that stimulate the thyroid. In cases where TRAb testing is inconclusive or unavailable, a radioactive iodine uptake (RAIU) scan provides functional imaging. A diffusely high uptake is characteristic of Graves' disease, distinguishing it from the patchy uptake of toxic nodules or the very low uptake seen in thyroiditis. This step is vital for guiding appropriate and etiology-specific treatment.
The field of Graves' disease diagnostics is advancing beyond simple confirmation of disease, with modern technology and a deeper understanding of biomarkers enhancing patient management across the entire care continuum. This evolution focuses on leveraging diagnostic tools not just for initial identification, but also for personalizing treatment strategies and improving long-term outcomes.
Laboratory biomarkers, particularly TSH receptor antibodies (TRAb), now play a critical role in prognosis and monitoring. Their utility extends to predicting relapse risk after anti-thyroid drug therapy, assessing the likelihood of fetal or neonatal thyroid dysfunction in pregnant patients, and offering an objective measure of disease activity alongside clinical symptoms.

Advancements in immunoassay technology form the backbone of modern diagnostics. Automated, high-precision platforms now enable the rapid and reliable measurement of key biomarkers—from the ultra-sensitive TSH assays essential for screening to the specific TRAb tests required for definitive diagnosis—directly supporting the integrated, data-driven clinical pathway.
Alta DiagnoTech provides a comprehensive in vitro diagnostic (IVD) portfolio that spans the entire clinical pathway for Graves' disease, from initial screening to definitive diagnosis and treatment monitoring. Our solutions are built on advanced immunoassay platforms designed to deliver high sensitivity, specificity, and automation efficiency, empowering clinical laboratories to deliver accurate and timely results that are critical for patient management. If you have related needs, please feel free to contact us for more information or product support.
| Product Name | Technology | Application |
| Ultra-Sensitive TSH Immunoassay Kit | Chemiluminescent Microparticle Immunoassay (CMIA) | Initial Screening & Diagnosis: The primary test for detecting thyrotoxicosis. A suppressed result (<0.1 µU/mL) is a key initial indicator. |
| Free T4 (FT4) & Free T3 (FT3) Immunoassay Kits | Chemiluminescent Immunoassay (CLIA) | Confirming & Stratifying Thyrotoxicosis: Quantitative measurement of free thyroid hormones to confirm diagnosis and assess severity. Essential for detecting T3 toxicosis. |
| TSH Receptor Antibody (TRAb) Assay Kit | Third-Generation Automated Binding Immunoassay | Differential Diagnosis & Confirmation: The gold-standard test for specifically confirming Graves' disease by detecting pathogenic stimulating or blocking antibodies. Also used for prognosis and monitoring. |
| Thyroid Peroxidase Antibody (TPOAb) Assay Kit | Automated Chemiluminescent Immunoassay | Supportive Autoimmune Marker: Aids in assessing general thyroid autoimmunity. Highly prevalent in Graves' disease and useful in differential diagnosis, particularly when TRAb is negative. |
| Automated Immunoassay Analyzer | Modular, High-Throughput Immunoassay System | Integrated Testing Platform: A scalable instrument system designed to run the entire thyroid diagnostic menu with walk-away automation, ensuring workflow efficiency and consistent results. |
Reference
This article is for research use only. Do not use in any diagnostic or therapeutic application.
|
There is no product in your cart. |