- Home
- Resource
- Explore & Learn
- Detection of Diverse HIV Strains by the m-PIMATM HIV-1/2 Detect Point-of-Care Assay
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Human Immunodeficiency Virus (HIV) is a highly diverse and mutable virus, presenting significant challenges for diagnostic assays. HIV-1, the predominant type, is categorized into four groups (M, N, O, and P), with Group M further divided into nine subtypes (A-D, F-H, J, K) and numerous circulating recombinant forms (CRFs) and unique recombinant forms (URFs). HIV-2 also exhibits substantial diversity across its subtypes (A-H) and CRFs. This genetic complexity poses a significant challenge for diagnostic assays, which often rely on detecting conserved sequences within the viral genome. Traditional diagnostic methods may fail to detect rare or divergent strains, leading to false negatives and missed diagnoses.
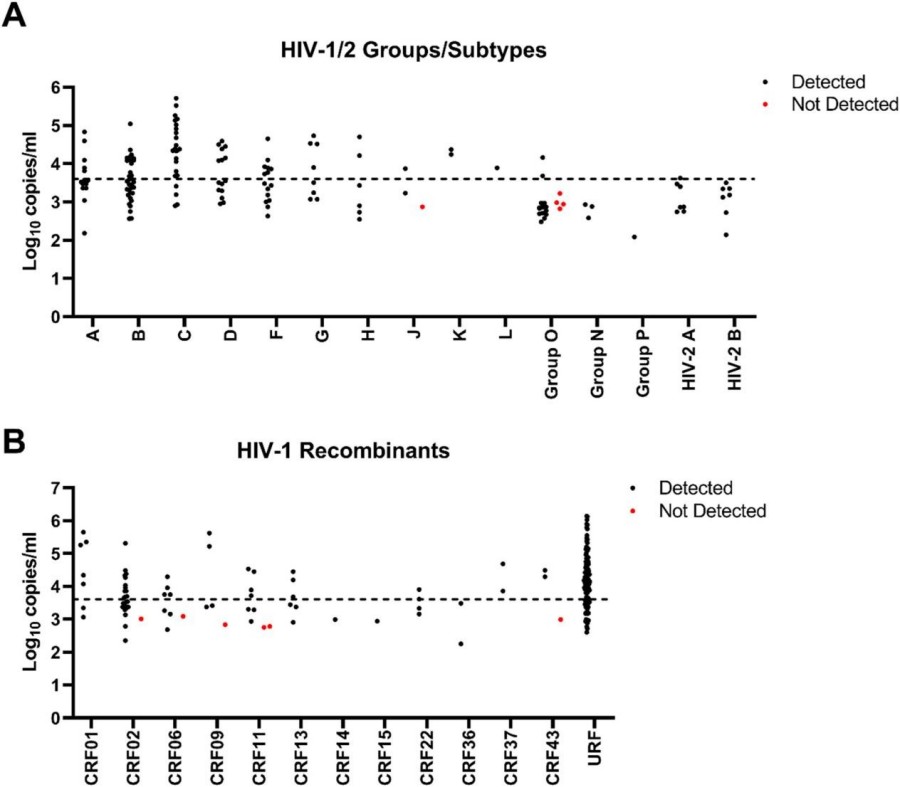 Fig.1 Performance of m-PIMA HIV-1/2 Detect on a challenge panel consisting of HIV-1/2 groups, subtypes, CRFs, and URFs (n = 340). (Anderson M., et al., 2025)
Fig.1 Performance of m-PIMA HIV-1/2 Detect on a challenge panel consisting of HIV-1/2 groups, subtypes, CRFs, and URFs (n = 340). (Anderson M., et al., 2025)
The m-PIMA HIV-1/2 Detect assay, developed by Abbott Diagnostics, represents a significant advancement in HIV diagnostics. This qualitative point-of-care (POC) multiplex RT-PCR assay is designed to detect HIV-1 and HIV-2 RNA in whole blood and plasma samples. The assay targets multiple regions of the viral genome, including the 5' LTR, gag, and pol regions of HIV-1, and the 5' LTR region of HIV-2. By using eight primers and probes for HIV-1 and five for HIV-2, the m-PIMA HIV-1/2 Detect assay can detect a wide range of viral strains with high sensitivity and specificity.
To evaluate the effectiveness of the m-PIMA HIV-1/2 Detect assay, a comprehensive study was conducted using a diverse panel of 340 serum/plasma specimens and diluted cultured virus isolates. This panel included representatives of HIV-1 groups M, N, O, P, various subtypes, CRFs, URFs, and HIV-2. The study also included an in silico analysis of 53,503 HIV-1 and 68 HIV-2 sequences from the National Center for Biotechnology Information (NCBI) database to predict the assay's performance against a broader range of circulating strains.

The results of the study demonstrated the m-PIMA HIV-1/2 Detect assay's exceptional ability to detect a wide range of HIV strains. The assay detected HIV in 329 out of 340 tested samples (96.8%). Among samples with a viral load (VL) greater than 4000 copies/mL (the assay's design sensitivity), detection was 100%. Even at lower viral loads (between 3.0 and 3.6 log copies/mL), the assay detected 96.9% of samples. For samples with a VL below 3.0 log copies/mL, the detection rate was 87.3%. Importantly, the assay detected at least one member from each subtype/CRF and all URFs tested.
The in silico analysis further supported the assay's inclusivity. Out of 53,503 HIV-1 sequences analyzed, only 2 (0.0037%) had mutations that could potentially reduce detection below the 90% identity threshold. For HIV-2, only 1 out of 68 sequences (1.47%) had such mutations. This suggests that the m-PIMA HIV-1/2 Detect assay is highly effective in detecting the vast majority of circulating HIV strains.

The ability to detect a wide range of HIV strains is crucial for achieving the UNAIDS 95-95-95 goals, which aim to ensure that 95% of people with HIV know their status, 95% of those diagnosed receive antiretroviral therapy, and 95% of those on therapy achieve viral suppression. The m-PIMA HIV-1/2 Detect assay's high sensitivity and inclusivity make it a valuable tool for rapid, accurate diagnosis, especially in resource-limited settings where access to advanced diagnostic technologies may be limited.
The m-PIMA HIV-1/2 Detect assay not only addresses the challenge of HIV diversity but also offers significant advantages in terms of speed and accessibility. Its rapid turnaround time (results available within 70 minutes) and the ability to use small sample volumes (25 µL of whole blood or plasma) make it particularly suitable for point-of-care settings. This assay can be used for early infant diagnosis (EID), where timely detection is critical for initiating antiretroviral therapy and improving outcomes.
Moreover, the m-PIMA HIV-1/2 Detect assay's robust performance across diverse strains suggests that it can be a reliable tool for global HIV surveillance. By accurately detecting both common and rare variants, this assay can help monitor the spread of HIV and inform public health strategies. Future research should focus on expanding the sample size to include more rare subtypes and groups, as well as comparing the performance of the m-PIMA HIV-1/2 Detect assay with other diagnostic platforms.
The m-PIMA HIV-1/2 Detect assay represents a significant breakthrough in HIV diagnostics. Its ability to detect a wide range of HIV strains, including rare and divergent forms, makes it a powerful tool in the fight against HIV. With its rapid turnaround time and high sensitivity, this POC assay can help ensure that more people know their HIV status and receive the care they need. As researchers continue to monitor and address the evolving diversity of HIV, assays like the m-PIMA HIV-1/2 Detect will play a critical role in improving global health outcomes.
If you have related needs, please feel free to contact us for more information or product support.
Reference
This article is for research use only. Do not use in any diagnostic or therapeutic application.

Cat.No. GP-DQL-00203
Rotavirus Antigen Group A and Adenovirus Antigen Rapid Test Kit (Colloidal Gold)

Cat.No. GP-DQL-00206
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Card Style

Cat.No. GP-DQL-00207
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. GP-DQL-00211
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. GP-DQL-00212
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. IP-00189
Influenza A Rapid Assay Kit

Cat.No. GH-DQL-00200
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00201
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00202
Luteinizing Hormone Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00208
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. GH-DQL-00209
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit(Colloidal Gold), Strip Style

Cat.No. GH-DQL-00210
Luteinizing Hormone Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. IH-HYW-0001
hCG Pregnancy Test Strip

Cat.No. IH-HYW-0002
hCG Pregnancy Test Cassette

Cat.No. IH-HYW-0003
hCG Pregnancy Test Midstream

Cat.No. GD-QCY-0001
Cocaine (COC) Rapid Test Kit

Cat.No. GD-QCY-0002
Marijuana (THC) Rapid Test Kit

Cat.No. GD-QCY-0003
Morphine (MOP300) Rapid Test Kit

Cat.No. GD-QCY-0004
Methamphetamine (MET) Rapid Test Kit

Cat.No. GD-QCY-0005
Methylenedioxymethamphetamine ecstasy (MDMA) Rapid Test Kit

Cat.No. GD-QCY-0006
Amphetamine (AMP) Rapid Test Kit

Cat.No. GD-QCY-0007
Barbiturates (BAR) Rapid Test Kit

Cat.No. GD-QCY-0008
Benzodiazepines (BZO) Rapid Test Kit

Cat.No. GD-QCY-0009
Methadone (MTD) Rapid Test Kit

Cat.No. GD-QCY-0011
Opiate (OPI) Rapid Test Kit

Cat.No. ID-HYW-0002
Multi-Drug Test L-Cup, (5-16 Para)

Cat.No. ID-HYW-0005
Multi-Drug Rapid Test (Dipcard & Cup) with Fentanyl

Cat.No. ID-HYW-0006
Multi-Drug Rapid Test (Dipcard & Cup) without Fentanyl

Cat.No. ID-HYW-0007
Multi-Drug 2~14 Drugs Rapid Test (Dipstick & Dipcard & Cup)

Cat.No. ID-HYW-0008
Fentanyl (FYL) Rapid Test (For Prescription Use)

Cat.No. ID-HYW-0009
Fentanyl Urine Test Cassette (CLIA Waived)

Cat.No. ID-HYW-0010
Fentanyl Urine Test Cassette (Home Use)
|
There is no product in your cart. |