- Home
- Resource
- Explore & Learn
- Designer Clearing Agents for Targeted Depletion of Antigen-Specific Antibodies
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Autoimmune diseases, characterized by the immune system's erroneous attack on self-tissues, pose significant challenges to global healthcare. Conditions such as rheumatoid arthritis, multiple sclerosis (MS), and systemic lupus erythematosus (SLE) affect millions worldwide, leading to chronic pain, disability, and reduced quality of life. Traditional treatments, primarily focusing on broad-spectrum immunosuppression, have provided symptomatic relief but come with substantial side effects, including increased susceptibility to infections and malignancies. The quest for more targeted and safer therapies has driven innovative research, leading to the advent of Seldegs—engineered clearing agents designed for the selective depletion of antigen-specific antibodies.
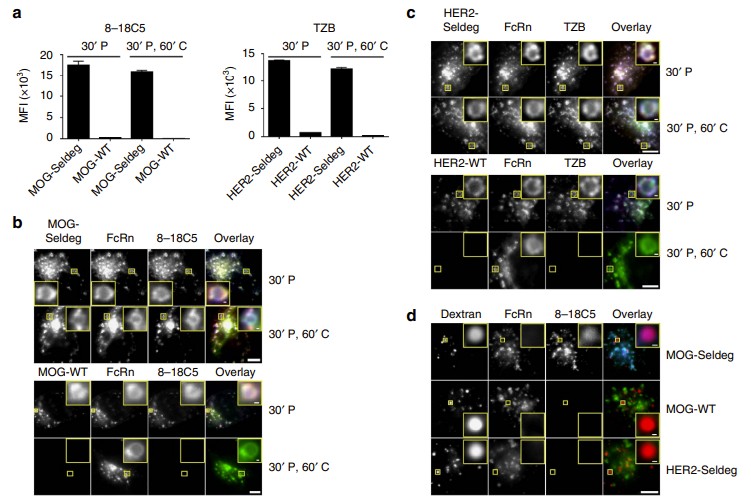 Fig.1 Seldegs increase the accumulation of antigen-specific antibodies in human endothelial (HMEC-1) cells expressing FcRn-GFP. (Devanaboyina S. C., et al., 2017)
Fig.1 Seldegs increase the accumulation of antigen-specific antibodies in human endothelial (HMEC-1) cells expressing FcRn-GFP. (Devanaboyina S. C., et al., 2017)
Autoimmune diseases arise from a complex interplay of genetic predisposition, environmental triggers, and dysregulation of the immune system. In these conditions, autoreactive B cells produce antibodies against self-antigens, leading to the formation of immune complexes that deposit in tissues, causing inflammation and damage. For instance, in MS, myelin oligodendrocyte glycoprotein (MOG)-specific antibodies attack the myelin sheath surrounding nerve fibers, disrupting neural transmission and causing neurological deficits. Similarly, in rheumatoid arthritis, antibodies against citrullinated proteins contribute to joint inflammation and destruction.
The persistence of these pathogenic antibodies necessitates long-term immunosuppressive therapy, which, while effective in managing symptoms, fails to address the root cause of the disease. Moreover, the nonselective nature of these treatments often leads to off-target effects, compromising the patient's ability to mount protective immune responses against pathogens.
Seldegs, short for selective degradation agents, represent a groundbreaking approach to autoimmune therapy. These engineered antibody-based reagents are designed to specifically target and deplete antigen-specific antibodies without affecting the levels of other, protective antibodies. The concept behind Seldegs stems from the understanding of the neonatal Fc receptor (FcRn), which plays a crucial role in regulating immunoglobulin G (IgG) levels and transport in the body.
Seldegs are constructed by fusing a recombinant antigen as a monomer to a dimeric, human IgG1-derived Fc fragment. This design ensures selective binding to the target antigen-specific antibodies while minimizing interactions with other IgG molecules. Mutations are introduced into the Fc fragment to enhance binding to FcRn at near-neutral pH, a property essential for the internalization and lysosomal degradation of the antibody-Seldeg complex within FcRn-expressing cells.
When administered, Seldegs selectively capture antigen-specific antibodies, forming complexes that are rapidly internalized into endothelial cells expressing FcRn. These complexes are then directed to lysosomes, where they undergo degradation, leading to a significant and rapid decrease in the levels of pathogenic antibodies. Importantly, Seldegs do not modulate the levels of other, non-targeted antibodies, preserving the patient's immune competence.
The efficacy of Seldegs has been demonstrated in preclinical studies using transgenic mouse models expressing human Fcγ receptors (huFcγR mice). In these models, Seldegs targeting MOG-specific antibodies in MS and HER2-specific antibodies in breast cancer (as a model for tumor-targeted therapy) were shown to induce rapid and substantial decreases in the levels of targeted antibodies.
For instance, in huFcγR mice injected with MOG-specific antibodies, subsequent administration of MOG-Seldeg resulted in a significant reduction in antibody levels in the blood and whole body within 24 hours. Notably, the total IgG levels remained unchanged, indicating the selectivity of Seldeg-mediated clearance. Similar results were observed with HER2-Seldeg, demonstrating the broad applicability of this approach.

The selective depletion of antigen-specific antibodies using Seldegs holds immense promise for the treatment of various autoimmune diseases and other conditions mediated by deleterious antibodies. By specifically targeting the pathogenic antibodies driving the autoimmune response, Seldegs offer a safer and more effective alternative to traditional immunosuppressive therapies.
In MS, Seldegs targeting MOG-specific antibodies could potentially halt the progression of the disease by preventing further demyelination and neural damage. Similarly, in rheumatoid arthritis, Seldegs designed to deplete antibodies against citrullinated proteins could alleviate joint inflammation and prevent structural damage. The ability to selectively remove pathogenic antibodies without compromising the entire immune system represents a significant advancement in the management of autoimmune diseases.
Beyond autoimmune diseases, Seldegs have potential applications in oncology, particularly in the context of diagnostic imaging and antibody-based therapies. For instance, HER2-specific antibodies are widely used the diagnosis and treatment of HER2-overexpressing breast cancer. However, the presence of background antibodies can interfere with diagnostic imaging and reduce the efficacy of targeted therapies. Seldegs targeting HER2 could selectively deplete these background antibodies, enhancing the accuracy of diagnostic imaging and improving the therapeutic outcome.
While the preclinical data on Seldegs are promising, several challenges remain to be addressed before their clinical translation. These include optimizing the pharmacokinetic properties of Seldegs to ensure prolonged circulation and efficient targeting, minimizing potential immunogenicity, and developing robust assays to monitor Seldegs levels and activity in patients.
If you have related needs, please feel free to contact us for more information or product support.
Reference
| Cat.No | Product Name | Price |
|---|---|---|
| AADA-HMM-0004 | Dextromethorphan (DXM)-02 | Add To Cart |
| AAKIM-HMM-0002 | Urinary Microalbumin (HSA[MAU])-04 | Add To Cart |
| AADA-HMM-0006 | Dextromethorphan (DXM)-40 | Add To Cart |
| AACB-HMM-0012 | Procalcitonin (PCT)-01 | Add To Cart |
| AACB-HMM-0004 | Heart-type Fatty Acid-bindin Protein (H-FABP)-01 | Add To Cart |
| AAID-HMM-0007 | Influenza A Virus (FluA)-09 | Add To Cart |
| AACB-HMM-0015 | Procalcitonin (PCT)-09 | Add To Cart |
| AALIM-HMM-0003 | Glycocholic Acid (CG) -98 | Add To Cart |
| AADA-HMM-0003 | Etomidate (ETO) | Add To Cart |
| AACB-HMM-0009 | D-Dimer-06 | Add To Cart |
| AACB-HMM-0010 | D-Dimer-10 | Add To Cart |
| AACB-HMM-0002 | N-terminal pro-brain natriuretic peptide (NT-ProBNP)-02 | Add To Cart |
| AATM-HMM-0005 | Carbohydrate Antigen 125 (CA125)-01 | Add To Cart |
| CLRM-HMM-0002 | DNP-BSA | Add To Cart |
| AATM-HMM-0003 | Ferritin-03 | Add To Cart |
This article is for research use only. Do not use in any diagnostic or therapeutic application.
|
There is no product in your cart. |