Pulmonary hypertension (PH) represents a complex hemodynamic disorder characterized by elevated blood pressure in the pulmonary circulation, leading to progressive right heart failure. This resource provides a comprehensive guide to the standardized diagnostic pathway for PH, detailing how laboratory algorithms and essential biomarkers work in concert with imaging techniques to accurately classify disease subtypes, assess disease severity, and guide targeted therapeutic interventions.
Overview of Pulmonary Hypertension
Pulmonary hypertension (PH) is a hemodynamic disorder defined by abnormally high blood pressure in the pulmonary arteries, which carry blood from the heart to the lungs. This condition imposes significant strain on the right ventricle of the heart, ultimately leading to right heart failure if left untreated. PH is not a single disease but is categorized into five major clinical groups based on underlying cause, ranging from pulmonary arterial vascular disease to conditions originating in the left heart or lungs. Its symptoms, such as shortness of breath, fatigue, and chest pain, are often non-specific, making accurate diagnosis dependent on a systematic and multi-modal approach.
555
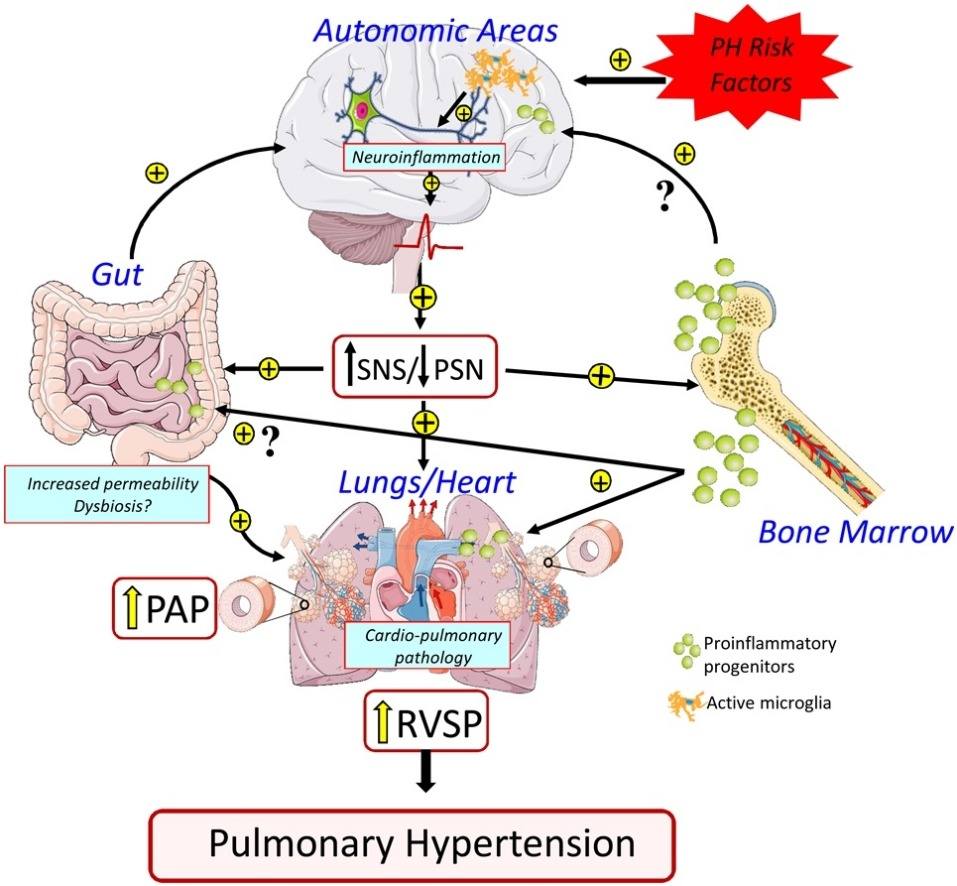 Fig.1 Pulmonary hypertension: pathophysiology beyond the lung. (Oliveira A C, et al., 2020)
Fig.1 Pulmonary hypertension: pathophysiology beyond the lung. (Oliveira A C, et al., 2020)
Standardized Diagnostic Pathway for Pulmonary Hypertension
The diagnosis of pulmonary hypertension (PH) follows a structured, multi-step pathway designed to progress from initial suspicion to definitive hemodynamic confirmation and etiological classification. This standardized approach is crucial for ensuring accurate diagnosis, appropriate treatment, and improved patient outcomes. Two investigative pillars form the core of this pathway: echocardiography as the primary non-invasive screening tool, and right heart catheterization as the indispensable gold standard for definitive diagnosis.
Echocardiography
Echocardiography serves as the fundamental initial imaging study in the evaluation of suspected PH. It provides a non-invasive estimate of pulmonary artery pressure and offers critical assessment of cardiac structure and function, including right atrial and ventricular size, wall thickness, and contractility. While it cannot definitively confirm PH, a negative echocardiogram makes the diagnosis highly unlikely, making it an excellent gatekeeper for further, more invasive testing.
Right Heart Catheterization (RHC)
Right heart catheterization (RHC) is the gold standard procedure required to definitively establish a diagnosis of PH. It involves directly measuring pressures within the right side of the heart and the pulmonary arteries. RHC provides precise hemodynamic data, including mean pulmonary arterial pressure (mPAP) and pulmonary vascular resistance (PVR), which are essential for both confirming the diagnosis and guiding subsequent treatment strategies.
Laboratory Algorithms for Pulmonary Hypertension Diagnostics
Laboratory testing provides a systematic framework for implementing the World Health Organization (WHO) classification system in pulmonary hypertension (PH). By following structured diagnostic algorithms, clinicians can objectively navigate the differential diagnosis to assign the correct WHO group. This methodical approach ensures comprehensive evaluation of potential etiologies while efficiently excluding alternative diagnoses, ultimately guiding targeted management strategies. The laboratory pathway particularly focuses on distinguishing among four key WHO groups through specific testing strategies.
Rule Out Group 4 (CTEPH)
Laboratory evaluation for chronic thromboembolic pulmonary hypertension centers on thrombophilia assessment. Testing for antiphospholipid antibodies, inherited thrombophilias, and D-dimer helps identify underlying hypercoagulable states that predispose to chronic thromboembolism. These investigations complement imaging findings to establish the diagnosis of CTEPH and inform long-term management decisions.
Identify Group 1 (PAH)
The diagnostic algorithm for pulmonary arterial hypertension requires comprehensive evaluation for associated conditions. Autoimmune serology (ANA, anti-Scl-70, anticentromere antibodies), HIV testing, liver function tests, and thyroid function assessments form the core laboratory panel. These tests systematically screen for connective tissue diseases, infectious etiologies, portopulmonary hypertension, and endocrine disorders that constitute WHO Group 1.
Differentiate Group 2 and Evaluate Group 3
Laboratory distinction between left heart disease (Group 2) and lung disease/hypoxia (Group 3) relies on biomarker profiling and gas exchange assessment. BNP/NT-proBNP and cardiac troponins indicate ventricular stress and myocardial injury characteristic of cardiac origins, while arterial blood gas analysis and polycythemia point to chronic hypoxemia from pulmonary pathologies. This biochemical profiling, combined with pulmonary function data, enables accurate group assignment between these common PH categories.
Essential Biomarkers for Pulmonary Hypertension Diagnostics
Beyond their role in initial diagnosis, specific biomarkers provide crucial objective data for assessing disease severity, predicting outcomes, and monitoring response to therapy in pulmonary hypertension. These laboratory values help clinicians move beyond symptomatic evaluation to make informed decisions about treatment intensity and timing of intervention, forming a cornerstone of modern, personalized pulmonary hypertension (PH) management.
- BNP and NT-proBNP: These natriuretic peptides serve as the cornerstone biomarkers for quantifying right ventricular wall stress and are strongly correlated with prognosis and treatment response.
- Cardiac Troponins: The detection of even low-level elevations using high-sensitivity assays indicates ongoing myocardial micro-injury and is a marker of higher risk.
- Renal Function Tests (Creatinine, eGFR): The presence of renal impairment, often manifesting as a cardio-renal syndrome, is a critical prognostic indicator and can influence therapeutic choices.
- Liver Function Tests (Albumin, Bilirubin, INR): Abnormalities reflect the consequences of right heart failure on hepatic congestion and synthetic function, providing insight into disease progression.
IVD Products for Pulmonary Hypertension
Alta DiagnoTech provides a comprehensive portfolio of advanced in vitro diagnostic (IVD) solutions to support the accurate diagnosis and ongoing management of pulmonary hypertension. Our innovative testing solutions deliver precise, actionable results that enable clinicians to stratify risk, monitor disease progression, and make informed treatment decisions throughout the patient care journey. If you have related needs, please feel free to contact us for more information or product support.
| Product Name |
Technology |
Application |
| High-Sensitivity Cardiac Troponin I Assay |
Chemiluminescent Immunoassay (CLIA) |
Quantitative detection of myocardial injury for risk stratification in PH patients. |
| BNP/NT-proBNP Test Kit |
Electrochemiluminescence Immunoassay (ECLIA) |
Assessment of right ventricular stress and heart failure severity for prognosis and treatment monitoring. |
| Autoimmune Antibody Panel |
Multiplex Immunoassay |
Simultaneous detection of autoantibodies (ANA, Anti-Scl-70, etc.) for identification of connective tissue disease-associated PAH. |
| Thrombophilia Screening Panel |
Chemiluminescent Immunoassay (CLIA) |
Comprehensive evaluation of antiphospholipid antibodies and inherited thrombophilias to support CTEPH diagnosis. |
| Liver and Renal Function Panel |
Photometric/Immunoturbidimetric Analysis |
Integrated assessment of hepatic and renal function to monitor PH complications and guide therapy. |
Reference
- Oliveira A C, Richards E M, Raizada M K. Pulmonary hypertension: pathophysiology beyond the lung[J]. Pharmacological research, 2020, 151: 104518.
This article is for research use only. Do not use in any diagnostic or therapeutic application.



 Fig.1 Pulmonary hypertension: pathophysiology beyond the lung. (Oliveira A C, et al., 2020)
Fig.1 Pulmonary hypertension: pathophysiology beyond the lung. (Oliveira A C, et al., 2020)