Decoding Clostridioides Difficile Infection (CDI): Key Biomarkers, Molecular Methods, and the Role of Toxin Detection
Clostridioides difficile infection (CDI) is a major cause of healthcare-associated diarrhea where accurate diagnosis is critical to distinguish active infection from harmless colonization. This resource provides a detailed guide to the core components of modern CDI diagnostics, covering the key biomarkers (Toxins A/B and GDH), explaining the technologies behind detection methods (EIA, PCR), and clarifying the pivotal role of toxin detection in clinical decision-making to ensure appropriate patient management.
Overview of Clostridioides difficile Infection
Clostridioides difficile Infection (CDI) is a leading cause of healthcare-associated infectious diarrhea, occurring primarily after antibiotic use disrupts the normal gut flora, allowing the spore-forming bacterium Clostridioides difficile to overgrow. The disease spectrum ranges from mild diarrhea to severe, life-threatening pseudomembranous colitis. Diagnosis is not based on symptoms alone and requires specific laboratory testing of stool samples to detect either the presence of the organism's toxins (A and B), which are the direct cause of intestinal damage, or the toxin gene (tcdB) that enables its production. Accurate diagnosis is crucial to guide appropriate treatment, prevent complications, and implement infection control measures.
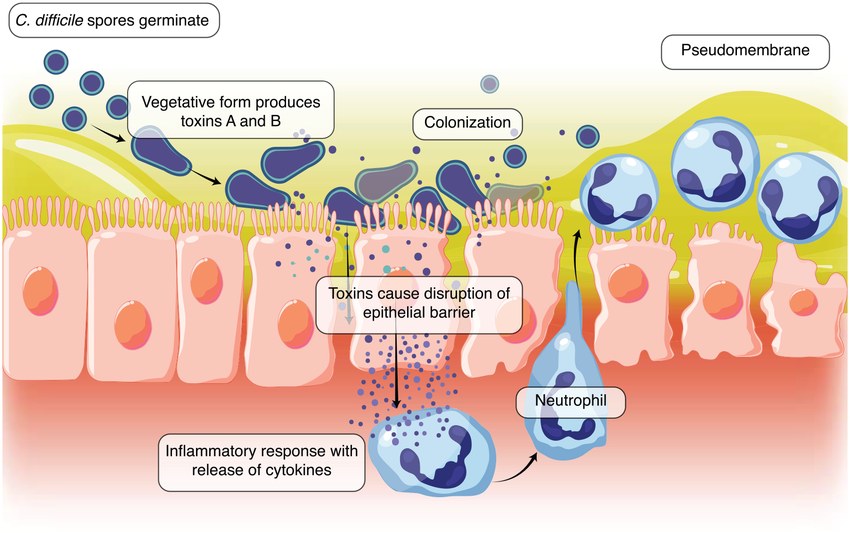 Fig.1 The pathogenesis of Clostridioides difficile infection (CDI). (Seekatz, Anna Maria, et al., 2022)
Fig.1 The pathogenesis of Clostridioides difficile infection (CDI). (Seekatz, Anna Maria, et al., 2022)
Biomarkers for Clostridioides difficile Infection Diagnostics
The accurate diagnosis of Clostridioides difficile infection (CDI) hinges on detecting specific biomarkers in stool samples that differentiate harmless colonization from active, toxin-mediated disease. Understanding the distinct roles of these biomarkers is fundamental to selecting the correct laboratory tests and interpreting their results.
Toxins A and B
Toxins A (TcdA) and B (TcdB) are the primary virulence factors directly responsible for the intestinal damage and clinical symptoms of CDI. Their detection in stool is considered the definitive evidence of active infection, as it confirms that the present C. difficile bacteria are not merely colonizing the gut but are producing the pathogenic toxins that cause disease.
Glutamate Dehydrogenase (GDH)
Glutamate dehydrogenase (GDH) is a constitutive enzyme produced by all C. difficile strains, both toxigenic and non-toxigenic. It serves as a highly sensitive screening marker for the presence of the bacterium itself. A positive GDH test indicates that C. difficile is present, but it cannot distinguish between colonization and active infection, as it does not confirm toxin production.
Diagnostic Methods for Clostridioides difficile Infection
Laboratory diagnosis of Clostridioides difficile infection (CDI) utilizes distinct methodological approaches, each with specific targets, performance characteristics, and clinical implications. Selecting the appropriate test or combination of tests is critical to accurately distinguish active infection from asymptomatic colonization.
Toxin Detection Methods
Toxin detection methods, such as enzyme immunoassays (EIAs), directly identify the presence of the pathogenic toxins A and B in stool samples. While these tests are rapid and confirm active toxin-mediated disease, their moderate sensitivity can lead to false-negative results if toxin levels are low. The historical gold standard, the cell culture cytotoxicity neutralization assay (CCNA), is highly specific but is labor-intensive and slow, making it unsuitable for routine clinical use.
Molecular Detection Methods
Molecular detection methods, primarily nucleic acid amplification tests (NAATs/PCR), target and amplify the bacterial gene (tcdB) responsible for toxin production. These tests offer excellent sensitivity and specificity for detecting the presence of a toxigenic C. difficile strain. However, their high sensitivity is a double-edged sword, as they may also detect asymptomatic carriers or residual genetic material after infection, potentially leading to the over-diagnosis of clinically irrelevant colonization.
The Critical Role of Toxin Detection in Clinical Decision-Making
Direct detection of C. difficile toxins (A and B) in stool is the definitive linchpin for confirming active infection and guiding appropriate treatment, as it distinguishes between asymptomatic colonization (presence of the bacterium or its genes) and clinically significant disease (actual toxin production causing intestinal damage). A positive toxin test strongly supports the diagnosis of CDI and justifies initiating specific antibiotic therapy, whereas reliance on highly sensitive molecular tests (like PCR) alone, without toxin confirmation, can lead to over-diagnosis and unnecessary treatment of patients who are merely carriers. Therefore, incorporating toxin detection into diagnostic algorithms is essential for accurate patient management, preventing antimicrobial misuse, and ensuring effective infection control.
IVD Products for Clostridioides Difficile Infection
With a focus on delivering precise and actionable results to address the critical challenge of distinguishing infection from colonization, Alta DiagnoTech provides comprehensive in vitro diagnostic (IVD) solutions for Clostridioides difficile Infection (CDI). Our portfolio supports efficient and accurate diagnostic pathways, from initial screening to definitive confirmation, by integrating key biomarker detection into streamlined workflows for clinical laboratories. If you have related needs, please feel free to contact us for more information or product support.
| Product Name |
Technology |
Application |
| Glutamate Dehydrogenase (GDH) Immunoassay Kit |
Enzyme Immunoassay (EIA) / Lateral Flow Immunochromatography |
High-throughput, rapid screening for the presence of C. difficile bacterium (both toxigenic and non-toxigenic strains) in stool samples. |
| Clostridium difficile Toxin A/B EIA Kit |
Enzyme Immunoassay (EIA) |
Direct detection of the pathogenic Toxin A and Toxin B proteins in stool to confirm active, toxin-producing CDI. |
| Automated Toxin A/B EIA Assay |
Chemiluminescent Immunoassay (CLIA) on Automated Platform |
High-sensitivity, automated quantitative or qualitative detection of Toxins A/B, offering improved workflow efficiency and consistency. |
| C. difficile Toxin B Gene (tcdB) PCR Assay |
Real-Time Polymerase Chain Reaction (PCR) |
Highly sensitive and specific detection of the gene responsible for Toxin B production, identifying the presence of a toxigenic C. difficile strain. |
| Integrated CDI Diagnostic Panel (GDH + Toxin A/B + PCR) |
Multiplex Nucleic Acid Amplification Test (NAAT) with Immunoassay Reflex |
Comprehensive testing solution that simultaneously detects GDH antigen, Toxins A/B, and the tcdB gene in a single cartridge or workflow, enabling algorithmic testing and result interpretation. |
Reference
- Seekatz, Anna Maria, Nasia Safdar, and Sahil Khanna. "The role of the gut microbiome in colonization resistance and recurrent Clostridioides difficile infection." Therapeutic Advances in Gastroenterology 15 (2022): 17562848221134396.
This article is for research use only. Do not use in any diagnostic or therapeutic application.



 Fig.1 The pathogenesis of Clostridioides difficile infection (CDI). (Seekatz, Anna Maria, et al., 2022)
Fig.1 The pathogenesis of Clostridioides difficile infection (CDI). (Seekatz, Anna Maria, et al., 2022)