- Home
- Resource
- Explore & Learn
- Current Advances in Biofabrication of Soft Microfluidic Systems for Diagnostics and Disease Modeling
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
The intersection of microfluidics and biofabrication is paving the way for transformative advancements in medicine. Soft microfluidic systems have emerged as a game-changing tool for disease modeling, diagnostics, and personalized healthcare. These technologies, which involve the precise manipulation of fluids at micro scales, provide an unprecedented opportunity to create dynamic, human-like tissue models that can replicate the complexities of the human body. By offering highly customizable, scalable, and reproducible platforms, soft microfluidics are expected to revolutionize the way we approach disease understanding, drug discovery, and therapeutic interventions.
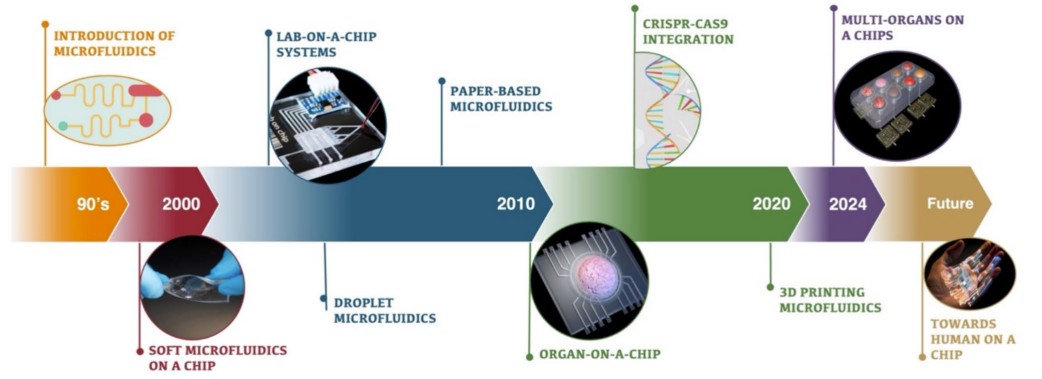 Fig.1 Evolution of microfuidics: from introduction to future innovations. (Casanova C. R., et al., 2024)
Fig.1 Evolution of microfuidics: from introduction to future innovations. (Casanova C. R., et al., 2024)
Microfluidic systems have existed for decades, initially finding applications in chemical and biological analysis. However, their integration into the healthcare industry is a relatively recent development. Early microfluidic systems were limited in scope, primarily used for diagnostic purposes such as point-of-care testing, lab-on-a-chip (LOC) systems, and DNA analysis. Over time, however, the field has evolved, and soft microfluidics has become a crucial component of modern biomedical engineering.
The shift to soft microfluidics has been driven by the ability to replicate complex biological environments more accurately than traditional in vitro systems. These systems use soft materials, often in combination with advanced biomaterials and bioinks, to create highly detailed and functional tissue models. The versatility of soft microfluidics allows for the simulation of various physiological and pathological conditions, offering profound insights into disease mechanisms and treatment responses.
Constructing Life-Like Tissue Models
Soft microfluidic systems are engineered to mimic the mechanical and biological properties of human tissues. By using advanced biomaterials such as hydrogels and stimuli-responsive bioinks, these systems recreate the cellular microenvironment with high precision. These systems can simulate blood flow, nutrient gradients, and oxygen diffusion, all of which are essential for maintaining tissue health and functionality in vitro.
The critical advantage of soft microfluidics over traditional cell culture techniques lies in their ability to replicate the dynamic and interactive nature of human tissues. By incorporating multiple cell types, recreating vascular networks, and allowing for mechanical manipulation of tissues, soft microfluidic systems provide a more accurate representation of how tissues behave in the human body. This level of detail is crucial for disease modeling, as it allows researchers to study the interaction between cells and their surrounding microenvironments under conditions that closely mirror those found in vivo.

Key Technologies Driving Soft Microfluidics
Soft microfluidic systems rely on several cutting-edge technologies to achieve their high level of precision and functionality. One of the most significant advancements has been the use of 3D bioprinting, which allows for the fabrication of complex tissue structures using bioinks that replicate the mechanical and biological properties of native tissues. These bioinks are typically made from natural materials such as collagen, alginate, or gelatin, and they can be tuned to support cell growth, differentiation, and interaction.
Additionally, soft lithography and laser ablation techniques have been utilized to create microfluidic channels and chambers with high resolution and precision. These methods allow for the fabrication of intricate structures, such as perfusable blood vessels, that are essential for creating functional organ models. The combination of these techniques enables the creation of highly complex in vitro models, from skin-on-a-chip devices to multi-organ-on-a-chip systems, which are pivotal for advancing personalized medicine.
One of the most promising applications of soft microfluidic systems is their ability to model complex diseases by recreating the tissue architecture and disease conditions. For example, cancer-on-a-chip models are gaining significant traction in oncology research. These devices allow researchers to simulate the tumor microenvironment, including the interactions between cancerous and non-cancerous cells, the extracellular matrix, and the vasculature. This enables the study of tumor progression, metastasis, and the effects of various therapies on cancer cells.
Moreover, soft microfluidic systems provide a platform for studying neurodegenerative diseases, such as Alzheimer's and Parkinson's. By integrating neurons, glial cells, and other supporting tissues in a microfluidic device, researchers can mimic the complex cellular interactions that occur in the brain. These systems help identify early biomarkers for neurodegenerative diseases and evaluate the effectiveness of potential drugs before they are tested in clinical trials.
Organs-on-chips have been heralded as one of the most revolutionary applications of soft microfluidic systems. These devices are designed to replicate the structure and function of human organs with remarkable precision, providing researchers with a tool to study diseases in a more human-relevant context. The lung-on-a-chip model, for example, mimics the alveolar-capillary barrier, which is critical for studying respiratory diseases such as asthma, pulmonary fibrosis, and COVID-19.
Another notable example is the skin-on-a-chip model, which replicates the full thickness of human skin, including the epidermis and dermis. This model is used to study skin diseases, wound healing, and drug absorption, offering a more accurate alternative to traditional animal testing. Furthermore, these systems are crucial for personalized medicine, as they allow researchers to test how individual patients' skin tissues react to different therapies.
Personalized medicine, which involves tailoring treatments to an individual's genetic makeup, environment, and lifestyle, is increasingly becoming a cornerstone of modern healthcare. Soft microfluidic systems are playing a key role in advancing this approach by providing a platform to create patient-specific disease models. These models are generated using cells or tissues from the patient, which are then incorporated into microfluidic devices that simulate their unique disease condition.
For example, in cancer therapy, soft microfluidic devices can be used to create a personalized tumor-on-a-chip model that mimics the specific tumor microenvironment of a patient. By testing various chemotherapeutic agents on the model, clinicians can identify which drugs are most likely to be effective for the patient, minimizing the risk of adverse side effects and improving treatment outcomes.
In the field of cardiovascular disease, researchers have developed vascular-on-a-chip models that replicate the human vasculature, allowing for the study of blood flow dynamics, vascular diseases, and the effects of different cardiovascular drugs. These models help clinicians understand how individual patients' vascular systems will respond to treatment, allowing for the optimization of therapies.
Soft microfluidic systems also have significant implications for drug screening. Their ability to create accurate, reproducible disease models allows for high-throughput screening of drug candidates, significantly speeding up the drug discovery process. Traditional drug testing methods often rely on animal models, which can be time-consuming, expensive, and not always representative of human biology. Soft microfluidic systems provide an alternative that is not only more efficient but also more ethical.
By integrating multiple cell types and simulating the complex tissue architecture of human organs, these systems enable the rapid evaluation of drug efficacy and toxicity. For example, a multi-organ-on-a-chip system can be used to test how a drug interacts with different tissues simultaneously, providing a more comprehensive understanding of its effects across the body. This reduces the reliance on animal testing and accelerates the development of new therapeutics.
Despite the significant advancements in soft microfluidic systems, several challenges remain. One of the primary obstacles is the replication of complex tissue structures. While current models can replicate basic tissue functions, many of the intricate features of human tissues—such as functional vasculature and immune cell interactions—remain difficult to reproduce. Progress in engineering functional endothelial networks, for example, is crucial for creating tissues that are truly capable of mimicking human organ function.
Additionally, scaling these systems for use in clinical settings remains a challenge. While soft microfluidics hold great promise for personalized healthcare, the scalability and standardization of these technologies are critical for their widespread adoption. Ensuring that these devices can be produced in large quantities, with consistent quality, will be essential for their implementation in healthcare.
Future developments in soft microfluidics will likely involve integrating newer technologies such as CRISPR gene editing and nanotechnology. By combining the precision of gene editing with the versatility of microfluidic platforms, researchers can create highly targeted therapies that are tailored to individual patients' genetic profiles. This integration will enable the development of personalized therapies that can correct genetic mutations at the source, with minimal off-target effects.
Moreover, the incorporation of artificial intelligence (AI) and machine learning (ML) into soft microfluidic systems holds tremendous potential. AI can be used to analyze the vast amounts of data generated by these systems, identifying patterns and making predictions about how different therapies will affect patients. This will further enhance the capabilities of soft microfluidics, making them a vital tool in precision medicine.
Soft microfluidic systems represent a remarkable leap forward in the field of disease modeling and personalized healthcare. By creating highly accurate, human-like models of diseases and organs, these systems offer new insights into disease mechanisms, therapeutic efficacy, and drug development. The integration of advanced biofabrication techniques, including 3D bioprinting and soft lithography, has paved the way for more sophisticated, customizable, and scalable platforms that can revolutionize medicine.
As research continues to evolve, soft microfluidic systems are set to play an increasingly important role in providing personalized, patient-specific treatments, offering hope for improved healthcare outcomes. With continued innovation, these technologies will not only improve the accuracy and efficiency of drug development but will also enable more tailored and effective therapies, marking a new era in medicine that is more personalized, efficient, and patient-centered.
If you have related needs, please feel free to contact us for more information or product support.
Reference
This article is for research use only. Do not use in any diagnostic or therapeutic application.

Cat.No. GP-DQL-00203
Rotavirus Antigen Group A and Adenovirus Antigen Rapid Test Kit (Colloidal Gold)

Cat.No. GP-DQL-00206
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Card Style

Cat.No. GP-DQL-00207
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. GP-DQL-00211
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. GP-DQL-00212
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. IP-00189
Influenza A Rapid Assay Kit

Cat.No. GH-DQL-00200
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold)
Cat.No. GH-DQL-00201
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00202
Luteinizing Hormone Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00208
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold), Strip Style
Cat.No. GH-DQL-00209
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit(Colloidal Gold), Strip Style

Cat.No. GH-DQL-00210
Luteinizing Hormone Rapid Test Kit (Colloidal Gold), Strip Style
Cat.No. IH-HYW-0001
hCG Pregnancy Test Strip
Cat.No. IH-HYW-0002
hCG Pregnancy Test Cassette
Cat.No. IH-HYW-0003
hCG Pregnancy Test Midstream

Cat.No. GD-QCY-0001
Cocaine (COC) Rapid Test Kit

Cat.No. GD-QCY-0002
Marijuana (THC) Rapid Test Kit
Cat.No. GD-QCY-0003
Morphine (MOP300) Rapid Test Kit
Cat.No. GD-QCY-0004
Methamphetamine (MET) Rapid Test Kit
Cat.No. GD-QCY-0005
Methylenedioxymethamphetamine ecstasy (MDMA) Rapid Test Kit
Cat.No. GD-QCY-0006
Amphetamine (AMP) Rapid Test Kit
Cat.No. GD-QCY-0007
Barbiturates (BAR) Rapid Test Kit

Cat.No. GD-QCY-0008
Benzodiazepines (BZO) Rapid Test Kit
Cat.No. GD-QCY-0009
Methadone (MTD) Rapid Test Kit
Cat.No. GD-QCY-0011
Opiate (OPI) Rapid Test Kit
Cat.No. ID-HYW-0002
Multi-Drug Test L-Cup, (5-16 Para)
Cat.No. ID-HYW-0005
Multi-Drug Rapid Test (Dipcard & Cup) with Fentanyl
Cat.No. ID-HYW-0006
Multi-Drug Rapid Test (Dipcard & Cup) without Fentanyl
Cat.No. ID-HYW-0007
Multi-Drug 2~14 Drugs Rapid Test (Dipstick & Dipcard & Cup)
Cat.No. ID-HYW-0008
Fentanyl (FYL) Rapid Test (For Prescription Use)
Cat.No. ID-HYW-0009
Fentanyl Urine Test Cassette (CLIA Waived)
Cat.No. ID-HYW-0010
Fentanyl Urine Test Cassette (Home Use)
|
There is no product in your cart. |