HPV Types and Cancer Link
There are over 200 known types of HPV, with about 40 associated with genital tract infections. Among them, 14 types are classified as high-risk (HR-HPV), including HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68. Persistent infection with HR-HPV is closely linked to the development of cervical cancer. For instance, HPV 16 and 18 alone are responsible for nearly 70% of cervical cancer cases, while HPV 45 and 31 contribute to about 5% and 10% respectively. In addition to cervical cancer, HR-HPV is also associated with 90% of anal cancers, 65% of vaginal cancers, 50% of vulvar cancers, 35% of penile cancers, and 60% of oropharyngeal cancers.



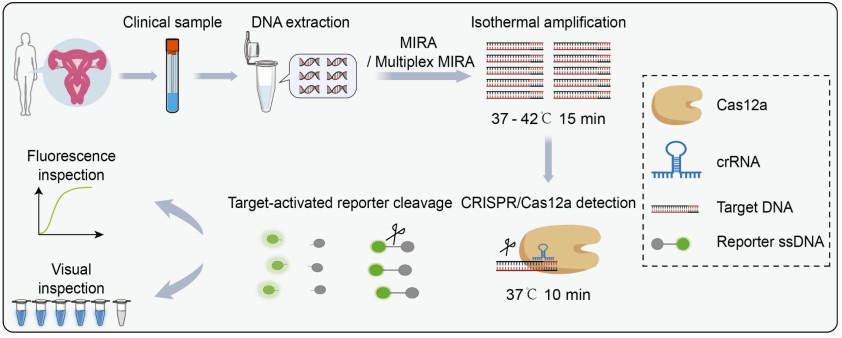 Fig.1 Illustration of the CRISPR/Cas12a-based fluorescent assay for HPV detection in clinical samples. (Yin L., et al., 2025)
Fig.1 Illustration of the CRISPR/Cas12a-based fluorescent assay for HPV detection in clinical samples. (Yin L., et al., 2025)