- Home
- Resource
- Explore & Learn
- Confounding Factors and Lesion Localization in the Validation of an In Vitro Diagnostic Test for Endometriosis
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Endometriosis, a chronic and estrogen-dependent disorder, affects approximately 10% of women of reproductive age globally. This condition is characterized by the presence of endometrium-like tissue outside the uterine cavity, most commonly in the pelvic region, including the ovaries, fallopian tubes, and peritoneum. The consequences of endometriosis are far-reaching, encompassing severe pelvic pain, dysmenorrhea, dyspareunia, and infertility.
The economic impact is also substantial. In the United States alone, the annual direct and indirect costs associated with endometriosis are estimated to be in the billions of dollars. These costs include medical treatments, lost productivity due to absenteeism, and reduced work efficiency. For instance, women with endometriosis often experience frequent sick days, especially during menstruation, when pain can be debilitating.
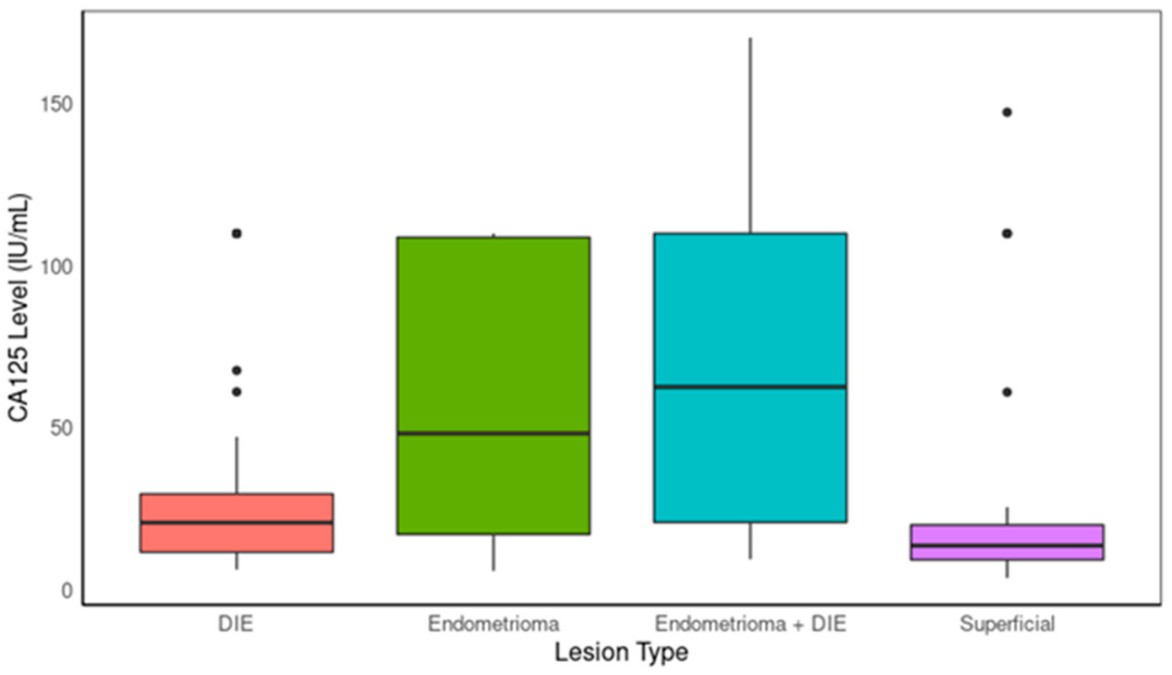 Fig.1 Boxplot comparing CA125 levels between lesion types. (Daoud E., et al., 2024)
Fig.1 Boxplot comparing CA125 levels between lesion types. (Daoud E., et al., 2024)
Historically, the gold standard for diagnosing endometriosis has been laparoscopy. This invasive surgical procedure involves making small incisions in the abdomen, inserting a laparoscope to visualize the pelvic organs, and taking tissue biopsies for histological examination. While laparoscopy provides definitive diagnostic information, it is associated with several drawbacks. It is costly, with expenses including surgical fees, anesthesia, and hospital stay. There are also risks involved, such as infection, bleeding, and damage to surrounding organs.
Imaging techniques, such as transvaginal ultrasound (TVUS) and magnetic resonance imaging (MRI), have been used as alternatives or adjuncts to laparoscopy. TVUS can detect some endometriotic lesions, especially larger ovarian endometriomas. However, it has limitations in detecting small, superficial, or deeply infiltrating lesions. MRI offers better soft-tissue contrast, but it is expensive, time-consuming, and may not be readily available in all healthcare settings. Moreover, both imaging methods have relatively low sensitivity and specificity for diagnosing early-stage endometriosis.
A new generation of non-invasive diagnostic tests for endometriosis is emerging, offering hope for earlier and more accessible diagnosis. These tests typically rely on the measurement of biomarkers in biological fluids, such as blood or urine, and the integration of clinical data.
One such promising approach combines the measurement of two serum biomarkers, CA125 and BDNF, with six clinical variables. CA125, a glycoprotein, has long been associated with endometriosis and other gynecological conditions. Elevated levels of CA125 are often found in women with endometriosis, especially in more advanced stages. BDNF (Brain-Derived Neurotrophic Factor), involved in neuronal survival, differentiation, and synaptic plasticity, has also been linked to the pathophysiology of endometriosis, particularly in pain perception.

The six clinical variables include factors such as a history of endometriosis-related surgery, the presence of painful periods as a primary symptom, the severity of menstrual pain in the previous cycle, the age at onset of intercourse-related pain, the age when regular painkiller use began, and the age at first diagnosis of an ovarian cyst. These variables are integrated into a specialized algorithm to generate a diagnostic result.

Clinical studies have shown varying performance of non-invasive tests across different types of endometriosis. For endometriomas, which are ovarian cysts filled with old blood and endometrial tissue, non-invasive tests have demonstrated relatively good sensitivity. In some studies, the combination of biomarkers and clinical variables has been able to detect a significant proportion of endometrioma cases.
For deep infiltrative endometriosis (DIE), which involves the growth of endometrial tissue deep into the pelvic organs and surrounding structures, the performance is also promising. DIE can cause severe pain and functional impairment. Non-invasive tests have shown the ability to identify many DIE cases, especially when considering the combination of biomarker levels and relevant clinical information.
However, for superficial endometriosis, which may consist of small, flat patches of endometrial tissue on the pelvic peritoneum, the sensitivity of non-invasive tests is still relatively lower. Nevertheless, these tests still outperform some traditional imaging methods in detecting these early-stage and often-overlooked lesions.
One of the most significant advantages of non-invasive testing is patient acceptance. Unlike laparoscopy, which requires surgery and anesthesia, non-invasive tests only involve a simple blood draw or urine collection. This reduces patient anxiety and discomfort, and also eliminates the risks associated with invasive procedures.
Non-invasive tests are also more cost-effective. They can be performed in outpatient settings, reducing the need for hospital admissions and associated costs. In addition, the ability to screen for endometriosis in a non-invasive manner can lead to earlier diagnosis, which may prevent the progression of the disease and reduce the need for more extensive and expensive treatments in the future.

Another advantage is the potential for early detection. By identifying endometriosis at an earlier stage, appropriate treatment can be initiated promptly. This can improve patient outcomes, such as reducing pain symptoms, improving fertility, and preventing the development of more severe complications.
The development of non-invasive diagnostic tests for endometriosis is an exciting area of research. Continued efforts are needed to improve the sensitivity and specificity of these tests, especially for detecting early-stage and superficial endometriosis.
Future research may focus on identifying additional biomarkers that can enhance the diagnostic accuracy of non-invasive tests. Combining multiple biomarkers from different biological pathways may provide a more comprehensive picture of the disease. Additionally, the integration of advanced technologies, such as machine learning algorithms, can further optimize the diagnostic models based on biomarker and clinical data.
As non-invasive tests become more refined and validated, they have the potential to become an integral part of the diagnostic algorithm for endometriosis. This could revolutionize the way endometriosis is diagnosed, leading to earlier detection, more personalized treatment, and ultimately, improved quality of life for women affected by this condition.
If you have related needs, please feel free to contact us for more information or product support.
Reference
This article is for research use only. Do not use in any diagnostic or therapeutic application.

Cat.No. GP-DQL-00203
Rotavirus Antigen Group A and Adenovirus Antigen Rapid Test Kit (Colloidal Gold)

Cat.No. GP-DQL-00206
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Card Style

Cat.No. GP-DQL-00207
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. GP-DQL-00211
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. GP-DQL-00212
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. IP-00189
Influenza A Rapid Assay Kit

Cat.No. GH-DQL-00200
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00201
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00202
Luteinizing Hormone Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00208
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. GH-DQL-00209
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit(Colloidal Gold), Strip Style

Cat.No. GH-DQL-00210
Luteinizing Hormone Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. IH-HYW-0001
hCG Pregnancy Test Strip

Cat.No. IH-HYW-0002
hCG Pregnancy Test Cassette

Cat.No. IH-HYW-0003
hCG Pregnancy Test Midstream

Cat.No. GD-QCY-0001
Cocaine (COC) Rapid Test Kit

Cat.No. GD-QCY-0002
Marijuana (THC) Rapid Test Kit

Cat.No. GD-QCY-0003
Morphine (MOP300) Rapid Test Kit

Cat.No. GD-QCY-0004
Methamphetamine (MET) Rapid Test Kit

Cat.No. GD-QCY-0005
Methylenedioxymethamphetamine ecstasy (MDMA) Rapid Test Kit

Cat.No. GD-QCY-0006
Amphetamine (AMP) Rapid Test Kit

Cat.No. GD-QCY-0007
Barbiturates (BAR) Rapid Test Kit

Cat.No. GD-QCY-0008
Benzodiazepines (BZO) Rapid Test Kit

Cat.No. GD-QCY-0009
Methadone (MTD) Rapid Test Kit

Cat.No. GD-QCY-0011
Opiate (OPI) Rapid Test Kit

Cat.No. ID-HYW-0002
Multi-Drug Test L-Cup, (5-16 Para)

Cat.No. ID-HYW-0005
Multi-Drug Rapid Test (Dipcard & Cup) with Fentanyl

Cat.No. ID-HYW-0006
Multi-Drug Rapid Test (Dipcard & Cup) without Fentanyl

Cat.No. ID-HYW-0007
Multi-Drug 2~14 Drugs Rapid Test (Dipstick & Dipcard & Cup)

Cat.No. ID-HYW-0008
Fentanyl (FYL) Rapid Test (For Prescription Use)

Cat.No. ID-HYW-0009
Fentanyl Urine Test Cassette (CLIA Waived)

Cat.No. ID-HYW-0010
Fentanyl Urine Test Cassette (Home Use)
|
There is no product in your cart. |