- Home
- Resource
- Explore & Learn
- Comprehensive Review of the PD-L1 SP263 Assay in Cancer Immunotherapy
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Cancer treatments have dramatically evolved over the past few decades. Historically, surgery, chemotherapy, and radiation therapy were the primary options for treating cancer. However, advancements in immunotherapy have significantly changed the treatment landscape, particularly in cancers that are resistant to traditional therapies. One of the most pivotal breakthroughs in immunotherapy is the use of immune checkpoint inhibitors (ICIs), which have shown remarkable efficacy in treating various cancers. These drugs work by blocking the interaction between PD-1 (programmed cell death protein 1) on T-cells and PD-L1 (programmed death ligand 1) on tumor cells, thereby unleashing the immune system's ability to attack and destroy cancer cells.
In this context, the PD-L1 SP263 assay has become an essential diagnostic tool for assessing PD-L1 expression in tumors. This assay helps determine which patients are most likely to benefit from PD-1/PD-L1-targeted immunotherapy. Through precise measurement of PD-L1 levels in tumor cells, clinicians can make more informed decisions, optimizing treatment outcomes and minimizing unnecessary side effects.
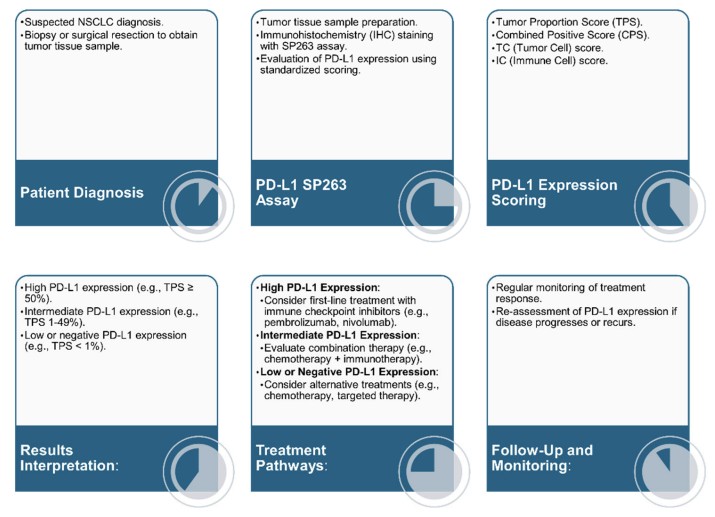 Fig.1 Process of PD-L1 testing using the SP263 assay and subsequent treatment pathways based on PD-L1 expression levels. (Pai T., et al., 2024)
Fig.1 Process of PD-L1 testing using the SP263 assay and subsequent treatment pathways based on PD-L1 expression levels. (Pai T., et al., 2024)
The PD-L1 SP263 assay is an immunohistochemical (IHC) diagnostic test used to detect the presence and level of PD-L1 expression in tumor tissues. PD-L1 is a transmembrane protein found on the surface of tumor cells and immune cells. Its role is to inhibit the immune system's response to cancer, preventing T-cells from attacking the tumor. By measuring the levels of PD-L1 expression on tumor cells, the SP263 assay helps to identify patients whose tumors may respond to immunotherapy.
The assay uses a monoclonal antibody, SP263, that binds specifically to PD-L1, allowing pathologists to assess the extent of PD-L1 expression in tissue samples. The Ventana platform is the most common platform used for performing the SP263 assay, and it has been approved by both the U.S. Food and Drug Administration (FDA) and the European Conformité Européenne (CE-IVD) for clinical use in various cancers, including non-small cell lung cancer (NSCLC).
The SP263 assay involves several critical steps:
The expression of PD-L1 on tumor cells is a key predictor of a patient’s response to immune checkpoint inhibitors. In cancers such as NSCLC, melanoma, head and neck squamous cell carcinoma (HNSCC), and gastric cancer, high levels of PD-L1 expression have been associated with a better response to PD-1/PD-L1 inhibitors like pembrolizumab, nivolumab, and atezolizumab.
High PD-L1 expression indicates that the tumor is actively suppressing the immune system, making it a prime target for therapies that block this pathway. On the other hand, tumors with low or absent PD-L1 expression are less likely to respond to these treatments. Thus, measuring PD-L1 levels helps identify patients who are most likely to benefit from these immunotherapies, facilitating personalized treatment approaches.

Clinical trials have consistently shown that PD-L1 expression plays a crucial role in the efficacy of immunotherapies. The KEYNOTE-024 trial, for example, demonstrated that pembrolizumab significantly improved survival in patients with advanced NSCLC who had high PD-L1 expression (≥50%). Similarly, the IMpower110 study showed that atezolizumab provided significant survival benefits in patients with NSCLC who had high PD-L1 expression as measured by the SP263 assay.
These studies have reinforced the importance of PD-L1 as a predictive biomarker for immunotherapy response, and they underscore the value of the PD-L1 SP263 assay in clinical practice.
NSCLC is the leading cause of cancer-related deaths worldwide, with a poor prognosis in advanced stages. Traditional treatment options, including surgery, chemotherapy, and radiation, have limited success in metastatic disease. However, immune checkpoint inhibitors targeting the PD-1/PD-L1 pathway have revolutionized the treatment of NSCLC.
The PD-L1 SP263 assay plays a central role in the management of NSCLC by identifying patients who are most likely to benefit from PD-1/PD-L1 inhibitors. The IMpower150 trial, for example, showed that atezolizumab, in combination with chemotherapy, improved overall survival (OS) and progression-free survival (PFS) in patients with metastatic NSCLC. The study utilized the PD-L1 SP263 assay to determine PD-L1 expression levels, demonstrating its clinical utility in guiding treatment decisions.
Several scoring systems are used to interpret PD-L1 expression levels, including:
The TPS is the most widely used scoring method in NSCLC, with a threshold of 50% considered high PD-L1 expression, which is associated with improved survival outcomes when treated with PD-1/PD-L1 inhibitors.

While the SP263 assay is a widely used tool for PD-L1 testing, it is not the only assay available. Other assays, such as the 22C3, 28-8, and SP142 assays, are also used in clinical practice. The 22C3 assay, for instance, is another FDA-approved assay used to measure PD-L1 expression in NSCLC.
Several studies have compared the SP263 assay with the 22C3 assay and found a high degree of concordance, particularly in NSCLC samples. For instance, a study found a Pearson correlation coefficient of 0.95 between the SP263 and 22C3 assays when used to assess PD-L1 expression in NSCLC samples. This high correlation suggests that the two assays can be used interchangeably, although the choice of assay may depend on factors like availability of testing platforms and specific clinical context.
However, differences in immune cell staining have been observed between the assays. The SP142 assay, for example, tends to detect fewer tumor cells than the SP263 assay, which may impact treatment decisions in certain patients.
The SP263 assay is not limited to NSCLC. Research has shown that it can be effectively used in a range of other cancers, including HNSCC, gastric cancer, melanoma, and renal cell carcinoma. For example, studies have demonstrated the utility of the SP263 assay in gastric cancer, where it showed high correlation with other PD-L1 assays like the 22C3 assay.
Similarly, in HNSCC, the SP263 assay has been shown to provide reliable results for PD-L1 testing, making it a valuable tool for clinicians managing this aggressive cancer type. The ability to detect PD-L1 expression in a wide range of cancers enhances the clinical relevance of the SP263 assay and supports its broader application in personalized cancer therapy.
One of the most exciting developments in the field of cancer diagnostics is the integration of artificial intelligence (AI) with traditional diagnostic tools. AI systems are being developed to enhance the accuracy and efficiency of PD-L1 testing. These AI-based models are capable of analyzing tissue samples and detecting PD-L1 expression with remarkable accuracy.
For instance, deep learning (DL) models have been shown to outperform pathologists in detecting PD-L1 expression in lung cancer samples, achieving accuracy rates above 96%. AI systems can also help reduce inter-observer variability, which is a known challenge in PD-L1 testing. By automating the detection and quantification of PD-L1, these AI models promise to improve the reliability of test results and streamline the diagnostic process.
As AI technologies continue to evolve, they are expected to become an integral part of the diagnostic workflow. AI-assisted PD-L1 testing could help pathologists analyze complex tissue samples more efficiently, leading to faster, more accurate diagnoses. Moreover, the integration of AI with existing platforms like the Ventana system could further enhance the clinical utility of the SP263 assay, making it even more accessible and effective in routine clinical practice.
The PD-L1 SP263 assay has proven to be an invaluable tool in the realm of cancer immunotherapy. Accurately measuring PD-L1 expression in tumors helps clinicians identify patients who are most likely to benefit from immune checkpoint inhibitors. The assay's widespread use in cancers like NSCLC, HNSCC, and gastric cancer has significantly improved the precision of immunotherapy, leading to better treatment outcomes and improved patient survival.
As research continues to explore new applications for the SP263 assay and the integration of artificial intelligence into diagnostic workflows, it is clear that this tool will play an increasingly important role in the fight against cancer. With its ability to guide personalized treatment decisions, the SP263 assay is set to remain at the forefront of cancer immunotherapy for years to come.
If you have related needs, please feel free to contact us for more information or product support.
Reference
This article is for research use only. Do not use in any diagnostic or therapeutic application.

Cat.No. GP-DQL-00203
Rotavirus Antigen Group A and Adenovirus Antigen Rapid Test Kit (Colloidal Gold)

Cat.No. GP-DQL-00206
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Card Style

Cat.No. GP-DQL-00207
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. GP-DQL-00211
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. GP-DQL-00212
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. IP-00189
Influenza A Rapid Assay Kit

Cat.No. GH-DQL-00200
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00201
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00202
Luteinizing Hormone Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00208
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. GH-DQL-00209
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit(Colloidal Gold), Strip Style

Cat.No. GH-DQL-00210
Luteinizing Hormone Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. IH-HYW-0001
hCG Pregnancy Test Strip

Cat.No. IH-HYW-0002
hCG Pregnancy Test Cassette

Cat.No. IH-HYW-0003
hCG Pregnancy Test Midstream

Cat.No. GD-QCY-0001
Cocaine (COC) Rapid Test Kit

Cat.No. GD-QCY-0002
Marijuana (THC) Rapid Test Kit

Cat.No. GD-QCY-0003
Morphine (MOP300) Rapid Test Kit

Cat.No. GD-QCY-0004
Methamphetamine (MET) Rapid Test Kit

Cat.No. GD-QCY-0005
Methylenedioxymethamphetamine ecstasy (MDMA) Rapid Test Kit

Cat.No. GD-QCY-0006
Amphetamine (AMP) Rapid Test Kit

Cat.No. GD-QCY-0007
Barbiturates (BAR) Rapid Test Kit

Cat.No. GD-QCY-0008
Benzodiazepines (BZO) Rapid Test Kit

Cat.No. GD-QCY-0009
Methadone (MTD) Rapid Test Kit

Cat.No. GD-QCY-0011
Opiate (OPI) Rapid Test Kit

Cat.No. ID-HYW-0002
Multi-Drug Test L-Cup, (5-16 Para)

Cat.No. ID-HYW-0005
Multi-Drug Rapid Test (Dipcard & Cup) with Fentanyl

Cat.No. ID-HYW-0006
Multi-Drug Rapid Test (Dipcard & Cup) without Fentanyl

Cat.No. ID-HYW-0007
Multi-Drug 2~14 Drugs Rapid Test (Dipstick & Dipcard & Cup)

Cat.No. ID-HYW-0008
Fentanyl (FYL) Rapid Test (For Prescription Use)

Cat.No. ID-HYW-0009
Fentanyl Urine Test Cassette (CLIA Waived)

Cat.No. ID-HYW-0010
Fentanyl Urine Test Cassette (Home Use)
|
There is no product in your cart. |