- Home
- Resource
- Disease Diagnosis
- Infectious Diseases
- Comprehensive Laboratory Testing for Hepatitis C: Screening, Diagnosis, and Beyond
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Hepatitis C virus (HCV) infection remains a significant global health challenge, with an estimated 58 million chronic cases worldwide. Accurate and timely diagnosis is critical for linking patients to life-saving antiviral treatments and achieving WHO's elimination targets. This resource provides a comprehensive overview of modern HCV diagnostic approaches, from serological screening to advanced molecular confirmation.
Hepatitis C is a bloodborne viral infection caused by the hepatitis C virus (HCV), which primarily targets the liver and can lead to chronic liver disease, cirrhosis, and hepatocellular carcinoma if left untreated. With an estimated 58 million people chronically infected worldwide (WHO, 2022), HCV often progresses silently, with many individuals remaining asymptomatic until advanced stages. Transmission occurs through exposure to infected blood, commonly via unsafe medical procedures, injection drug use, or contaminated blood products. While no vaccine exists, highly effective direct-acting antiviral (DAA) therapies can cure over 95% of infections.
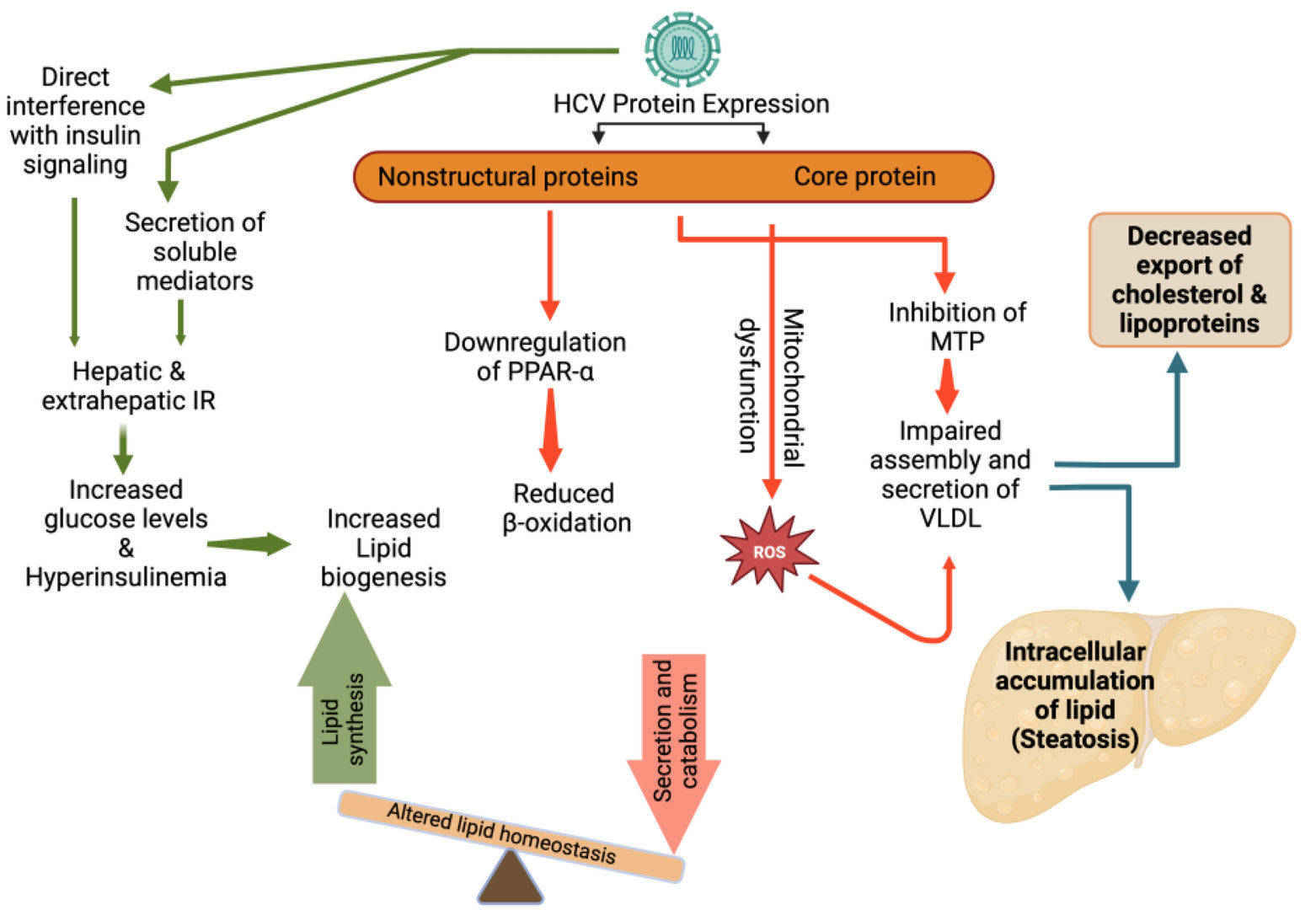 Fig.1 Hepatitis C virus (HCV) induced alterations in lipid metabolism and steatosis. (Elgretli W, et al., 2023)
Fig.1 Hepatitis C virus (HCV) induced alterations in lipid metabolism and steatosis. (Elgretli W, et al., 2023)
Screening for Hepatitis C is critical for early detection and intervention, as many infected individuals remain asymptomatic for years while the virus silently damages the liver. Identifying HCV early allows for timely treatment with highly effective direct-acting antivirals (DAAs), preventing progression to cirrhosis, liver cancer, and transmission to others. The CDC and WHO recommend priority screening for high-risk populations, including:
Serological testing for hepatitis C plays a pivotal role in screening and diagnosis by detecting anti-HCV antibodies, which indicate exposure to the virus. These tests are widely used due to their high sensitivity, cost-effectiveness, and scalability, making them essential for identifying at-risk populations and guiding further diagnostic steps.

The enzyme-linked immunosorbent assay (ELISA) or enzyme immunoassay (EIA) is the gold standard for HCV antibody detection in clinical laboratories. These tests offer exceptional sensitivity (>99%) and specificity (~99%), reliably identifying anti-HCV antibodies as early as 8–12 weeks post-infection. Modern 4th-generation ELISA assays can simultaneously detect antibodies and HCV core antigen, slightly reducing the window period. However, ELISA cannot differentiate between active, resolved, or past infections, necessitating confirmatory nucleic acid testing (NAT) for RNA detection.
Rapid point-of-care (POC) antibody tests provide results in 20–30 minutes using fingerstick blood, oral fluid, or serum, making them ideal for outreach programs or resource-limited settings. While convenient, these tests have lower sensitivity (∼90–95%) compared to ELISA, particularly in early infection or immunocompromised individuals.
While serological tests identify HCV exposure through antibodies, direct detection methods target viral components to confirm active infection, which are critical for diagnosis and treatment decisions. These approaches bypass the limitations of antibody testing and are indispensable for guiding antiviral therapy. Two key direct detection strategies include HCV core antigen (Ag) testing and HCV RNA testing.

HCV core antigen testing detects viral protein in blood, providing a cost-effective alternative to RNA testing with results available in 1–2 hours. It can identify active infection 1–2 weeks earlier than antibody tests, making it useful for screening and diagnosis in resource-limited settings, though it has slightly lower sensitivity (90-95%) than PCR for low viral loads (<3,000 IU/mL).

HCV RNA testing by PCR is the gold standard for confirming active infection, with ultra-sensitive detection (as early as 1-2 weeks post-exposure) and precise viral load quantification. It is essential for diagnosis, genotyping, and monitoring treatment response (e.g., SVR12), though requires specialized lab equipment and is more expensive than antigen testing.
Despite advances in HCV testing, significant challenges remain, including limited access to RNA testing in resource-limited settings, the high cost of NAT assays, and logistical barriers to linkage-to-care following screening. Future innovations aim to overcome these hurdles through point-of-care RNA tests and integration of AI to optimize testing algorithms. Additionally, non-invasive biomarkers (e.g., microRNAs) and multiplex platforms for simultaneous HCV/HIV/HBV screening are emerging to enhance early diagnosis and support global HCV elimination goals by 2030.
Alta DiagnoTech delivers end-to-end IVD solutions for hepatitis C, including high-performance test kits, diagnostic instruments, and consumables to support accurate screening and monitoring. If you have related needs, please feel free to contact us for more information or product support.
Reference
| Cat.No | Product Name | Price |
|---|---|---|
| BDDK-HMM-0003 | Hepatitis C Virus Nucleic Acid Test Kit (PCR-Fluorescent Probe Method) | Add To Cart |
| VI-QCY-0005 | Hepatitis C Virus (HCV) RT-PCR Test Kit | Add To Cart |
| NATR-HMM-0031 | Hepatitis C Virus Nucleic Acid Detection Reagent (RNA Pulldown) | Add To Cart |
This article is for research use only. Do not use in any diagnostic or therapeutic application.
|
There is no product in your cart. |