- Home
- Resource
- Explore & Learn
- Clinical Laboratory Approaches for In Vitro Chagas Disease Detection
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Chagas disease, caused by the protozoan parasite Trypanosoma cruzi, presents a dynamic clinical profile that complicates diagnostic efforts. The disease progresses through distinct phases, each with unique biological characteristics that demand tailored testing approaches. The acute phase, occurring 2-3 weeks post-infection, is marked by high parasitemia—elevated levels of parasites in the bloodstream—often accompanied by non-specific flu-like symptoms. In severe cases, particularly among immunosuppressed individuals or those infected via oral transmission, acute Chagas can lead to life-threatening myocarditis or fulminant disease. This phase transitions to a chronic stage after 8-12 weeks, where parasitemia declines significantly, and patients may remain asymptomatic for decades. Approximately 40% of chronic cases eventually develop cardiac complications, while a smaller subset experiences gastrointestinal manifestations such as megaesophagus or megacolon.
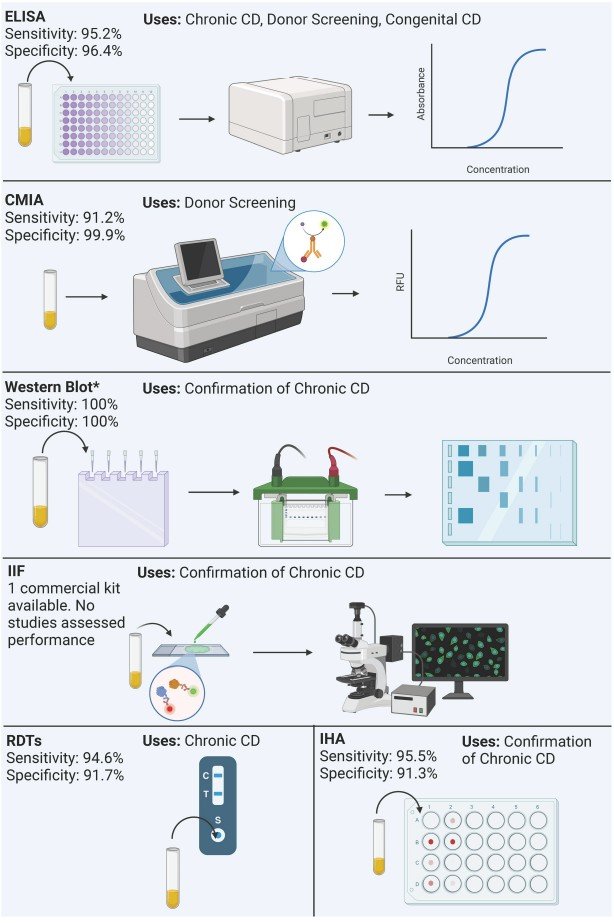 Fig.1 Summary of process and performance of serological methods included in the analysis. (Ascanio L. C., et al., 2024)
Fig.1 Summary of process and performance of serological methods included in the analysis. (Ascanio L. C., et al., 2024)
The parasite's genetic diversity further complicates diagnosis. T. cruzi exhibits at least seven discrete typing units (DTUs)—TcI to TcVI and Tcbat—each with distinct genetic and phenotypic traits. These variations can influence clinical presentation, immune response, and ultimately, the performance of diagnostic tests. For example, differences in parasite antigens across DTUs may affect the sensitivity of serological assays, which rely on detecting host antibodies to specific parasite proteins.
Transmission routes also impact diagnostic strategies. While vector-borne transmission via triatomine bugs remains most common in endemic regions, congenital transmission (from mother to child), transfusion-associated cases, and post-transplant reactivation require specialized testing protocols. Oral transmission, linked to contaminated food or beverages, presents unique challenges due to its potential for explosive outbreaks and severe acute manifestations.
Serological tests, which detect antibodies produced by the host in response to T. cruzi infection, form the backbone of chronic Chagas disease diagnosis. These assays leverage the immune system's robust and sustained response to the parasite during the chronic phase, where direct parasite detection becomes unreliable due to low parasitemia.
Enzyme-Linked Immunosorbent Assays (ELISA)

ELISAs dominate the serological landscape, with 48 distinct assays identified in clinical studies. These tests operate on the principle of antigen-antibody binding, using T. cruzi antigens (either recombinant proteins, lysates, or purified fractions) to capture specific antibodies in patient samples. The commercial ELISAs currently available demonstrate impressive performance metrics: sensitivity ranges from 77.4% to 100%, with specificities between 84.2% and 100%, and a mean sensitivity and specificity of 95.2% and 96.4%, respectively.
Notable commercial ELISAs include the Chagatest ELISA Recombinant v.3.0 (Wiener Laboratorios), approved by both the FDA and EU for donor screening and chronic disease diagnosis, with a sensitivity of 96.1% and specificity of 84.2%. Another standout is the Ortho Trypanosoma cruzi ELISA Test System (Ortho-Clinical Diagnostics), which achieves 96.4% sensitivity and 97.4% specificity, making it a staple in blood bank screening. The versatility of ELISAs is enhanced by their ability to incorporate various antigens—recombinant proteins, for example, often exhibit superior specificity by minimizing cross-reactivity with related parasites like Leishmania spp.
Despite their strengths, ELISAs have limitations. They require laboratory infrastructure, trained personnel, and longer turnaround times compared to point-of-care tests. Additionally, their performance can vary based on the antigen used and the population tested, with some assays showing reduced sensitivity in early chronic stages or among individuals with low antibody titers.
Rapid Diagnostic Tests (RDTs)
Rapid diagnostic tests, or immunochromatographic assays, address the need for point-of-care diagnosis in resource-limited settings. These user-friendly tests require minimal training and deliver results within 15–30 minutes, making them invaluable for field screening and endemic regions with limited laboratory access. Of the 17 RDTs identified in clinical evaluations, six remain commercially available, with performance metrics comparable to ELISAs for chronic disease: mean sensitivity of 94.66% and mean specificity of 91.73%.
The Chagas Detect Plus (InBios International) stands out as the only FDA-approved RDT, with 96.51% sensitivity and 93.6% specificity, approved for donor screening, congenital, and chronic disease diagnosis. The Chagas STAT PAK Assay (Chembio Diagnostic Systems) achieves 94.9% sensitivity and 98.23% specificity and is approved in the EU and Argentina. These tests rely on recombinant antigens, though proprietary formulations mean specific antigen details are often undisclosed.
RDTs face challenges, including declining market availability—only 35% of identified RDTs remain on the market—and variable performance in certain populations. For example, the TR Chagas Bio-Manguinhos (Bio-Manguinhos) exhibits 100% sensitivity but only 78.5% specificity, highlighting the need for careful selection based on local epidemiological context.
Other Serological Modalities

Complementary serological methods fill specific diagnostic niches. Chemiluminescent immunoassays (CMIAs) offer high throughput and automation, making them ideal for large-scale screening in blood banks. The Elecsys Chagas (Roche Diagnostics), approved by the FDA in 2024, demonstrates 98.45% sensitivity and 99.95% specificity, with applications in donor screening and congenital diagnosis. Indirect hemagglutination (IHA) assays, such as the Chagatest IHA (Wiener Laboratorios), use parasite-sensitized red blood cells to detect antibodies, achieving 88.9% sensitivity and 99.5% specificity for chronic disease and donor screening.
Western blot assays serve as confirmatory tools, with the Chagas Western Blot IgG assay (LDBio Diagnostics) showing 100% sensitivity and specificity for chronic Chagas. This EU-approved test uses native antigens from the TcVI DTU, aiding in distinguishing T. cruzi infections from cross-reactive Leishmania spp. Infections.
Molecular diagnostic methods, which detect T. cruzi DNA, are critical for acute infections, congenital cases, and reactivation in immunosuppressed patients—scenarios where parasitemia is sufficiently high to enable direct detection.
Polymerase Chain Reaction (PCR) Assays
PCR-based methods dominate molecular diagnostics, with 21 distinct assays identified, including conventional PCR (cPCR), real-time PCR (qPCR), and digital droplet PCR (ddPCR). These tests target repetitive DNA sequences in the parasite genome, such as kinetoplast DNA (kDNA) and nuclear satellite DNA (stDNA), to amplify and detect even minute quantities of parasite genetic material.

qPCR emerges as a balance between sensitivity, specificity, and practicality, with a mean sensitivity of 82.84% and specificity of 94.01%. It offers quantitative capabilities, enabling parasite load monitoring, valuable for assessing treatment response. The in-house qPCR developed by Colombia's Instituto Nacional de Salud achieves 79.95% sensitivity and 98.55% specificity, making it a regional standard for acute and chronic phase detection. ddPCR, a newer technology, demonstrates 100% sensitivity and specificity in limited studies, offering absolute quantification without standard curves, though its high cost limits widespread adoption.
cPCR, the most studied molecular method, has a lower mean sensitivity of 58.88% but remains useful in resource-limited settings due to its simplicity. Performance varies by target: assays targeting stDNA often outperform those using kDNA, particularly in chronic cases with low parasitemia.
Loop-Mediated Isothermal Amplification (LAMP)
LAMP offers a promising alternative to PCR, operating at constant temperatures (60–65°C) without a thermal cycler, reducing equipment requirements. This method amplifies DNA rapidly, delivering results in under an hour, with visual readout options (color change or fluorescence), eliminating the need for complex detection systems. LAMP assays targeting stDNA and 18S rRNA exhibit a mean sensitivity of 85.9% and specificity of 98.8%, with particular utility in congenital Chagas and field settings.
Despite its potential, LAMP faces availability challenges: prototype kits (e.g., Eiken Chemical's Loopamp) are no longer commercially available, limiting clinical adoption. Ongoing efforts to develop stable, affordable LAMP kits could expand access to molecular diagnostics in endemic regions.
The regulatory landscape for Chagas disease diagnostics is fragmented, with significant disparities between regions. As of 2024, the FDA has approved six commercial tests, all serological: three ELISAs, two CMIAs, and one RDT. Notably, no molecular tests are approved for in vitro diagnostics (IVD) in the United States, limiting options for acute and congenital cases.
In the EU, regulatory approval (CE marking) is more widespread, encompassing 10 ELISAs, four CMIAs, three RDTs, one Western blot, and three molecular tests. This broader approval reflects higher disease prevalence in neighboring endemic regions and proactive public health policies.
Endemic countries like Argentina and Brazil have robust local regulatory frameworks, supporting a range of tests tailored to regional needs. For example, Argentina approves multiple ELISAs, RDTs, and IHAs for IVD use, while Brazil's regulatory body clears tests like the Immuno-HAI Chagas (Wama Diagnóstica) for donor screening.
This patchwork of approvals creates challenges for global harmonization. Migrant populations in non-endemic countries may encounter delayed or inaccurate diagnosis due to limited access to appropriate tests, highlighting the need for standardized regulatory pathways and expanded test availability.
Diagnosing congenital Chagas requires specialized strategies, as maternal antibodies can persist in infants for up to 12 months, confounding serological tests. Molecular methods are preferred: qPCR and LAMP targeting stDNA achieve high sensitivity in newborns, with the Wiener Lab’s T. cruzi DNA test demonstrating 72.73% sensitivity and 99.15% specificity in congenital cases. Serological approaches, such as IgM-specific ELISAs or RDTs like Chagas Detect Plus, serve as complementary tools after the first few months of life.
Donor screening relies heavily on serological tests to prevent transmission via blood or organ transplants. ELISAs and CMIAs are preferred for their high throughput and reliability: the Ortho Trypanosoma cruzi ELISA and Elecsys Chagas are FDA-approved for this purpose, with sensitivities exceeding 96%. Molecular tests may be used adjunctively in high-risk donors or recipients, particularly in regions with high endemicity.
Oral transmission, linked to contaminated food, often causes severe acute Chagas with high parasitemia. Molecular tests (qPCR, LAMP) are critical for rapid diagnosis, enabling timely intervention. Serological tests may yield false negatives in the early acute phase due to delayed antibody production, making molecular methods indispensable in outbreak settings.
Despite advancements, Chagas disease diagnostics face significant hurdles. The lack of a universal gold standard complicates test validation, with varying reference standards across studies introducing bias. Molecular tests, while powerful for acute cases, remain limited by their reliance on parasitemia, which drops sharply in the chronic phase. Regulatory barriers and market forces have led to the discontinuation of promising tests, particularly RDTs and molecular assays, reducing access in endemic regions.
Future innovations should focus on multi-modal approaches: combining serological and molecular tests to cover all disease phases, developing pan-DTU antigens to address parasite diversity, and creating affordable point-of-care molecular assays. Additionally, biomarkers for treatment response are urgently needed, as current tests cannot reliably confirm parasite clearance post-therapy.
By addressing these gaps, the diagnostic community can improve early detection, reduce transmission, and ultimately mitigate the global burden of Chagas disease.
If you have related needs, please feel free to contact us for more information or product support.
Reference
This article is for research use only. Do not use in any diagnostic or therapeutic application.

Cat.No. GP-DQL-00203
Rotavirus Antigen Group A and Adenovirus Antigen Rapid Test Kit (Colloidal Gold)

Cat.No. GP-DQL-00206
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Card Style

Cat.No. GP-DQL-00207
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. GP-DQL-00211
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. GP-DQL-00212
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. IP-00189
Influenza A Rapid Assay Kit

Cat.No. GH-DQL-00200
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00201
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00202
Luteinizing Hormone Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00208
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. GH-DQL-00209
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit(Colloidal Gold), Strip Style

Cat.No. GH-DQL-00210
Luteinizing Hormone Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. IH-HYW-0001
hCG Pregnancy Test Strip

Cat.No. IH-HYW-0002
hCG Pregnancy Test Cassette

Cat.No. IH-HYW-0003
hCG Pregnancy Test Midstream

Cat.No. GD-QCY-0001
Cocaine (COC) Rapid Test Kit

Cat.No. GD-QCY-0002
Marijuana (THC) Rapid Test Kit

Cat.No. GD-QCY-0003
Morphine (MOP300) Rapid Test Kit

Cat.No. GD-QCY-0004
Methamphetamine (MET) Rapid Test Kit

Cat.No. GD-QCY-0005
Methylenedioxymethamphetamine ecstasy (MDMA) Rapid Test Kit

Cat.No. GD-QCY-0006
Amphetamine (AMP) Rapid Test Kit

Cat.No. GD-QCY-0007
Barbiturates (BAR) Rapid Test Kit

Cat.No. GD-QCY-0008
Benzodiazepines (BZO) Rapid Test Kit

Cat.No. GD-QCY-0009
Methadone (MTD) Rapid Test Kit

Cat.No. GD-QCY-0011
Opiate (OPI) Rapid Test Kit

Cat.No. ID-HYW-0002
Multi-Drug Test L-Cup, (5-16 Para)

Cat.No. ID-HYW-0005
Multi-Drug Rapid Test (Dipcard & Cup) with Fentanyl

Cat.No. ID-HYW-0006
Multi-Drug Rapid Test (Dipcard & Cup) without Fentanyl

Cat.No. ID-HYW-0007
Multi-Drug 2~14 Drugs Rapid Test (Dipstick & Dipcard & Cup)

Cat.No. ID-HYW-0008
Fentanyl (FYL) Rapid Test (For Prescription Use)

Cat.No. ID-HYW-0009
Fentanyl Urine Test Cassette (CLIA Waived)

Cat.No. ID-HYW-0010
Fentanyl Urine Test Cassette (Home Use)
|
There is no product in your cart. |