- Home
- Resource
- Explore & Learn
- Breakthrough in Liver Fibrosis Diagnosis: A New Non-Invasive LC-MS/MS Assay Kit
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Liver fibrosis is a progressive condition marked by the excessive accumulation of extracellular matrix proteins, resulting in the formation of scar tissue within the liver. It commonly stems from chronic liver injury due to viral hepatitis, alcohol abuse, non-alcoholic fatty liver disease (NAFLD), and other metabolic disorders. Early detection and intervention are crucial, as untreated liver fibrosis can progress to cirrhosis and liver failure. However, traditional diagnostic methods like liver biopsy are invasive, costly, and carry potential complications. Thus, the development of non-invasive diagnostic tools is essential to improve patient outcomes and reduce healthcare costs.
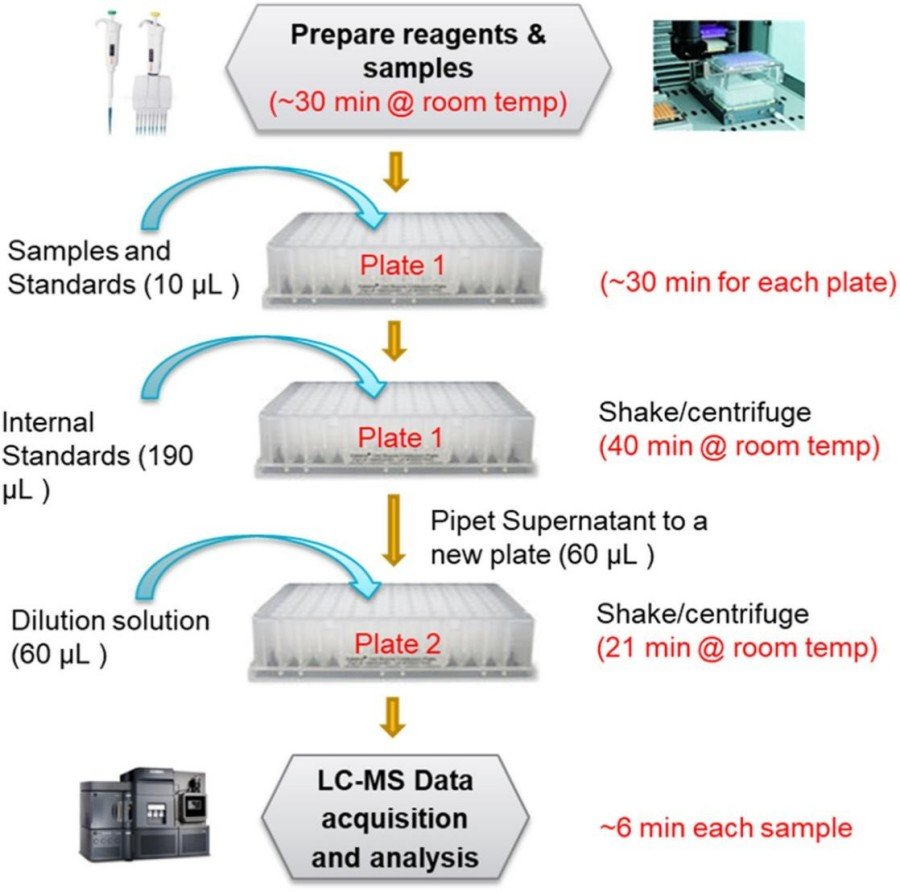 Fig.1 Sample analysis workflow. (Xie G., et al., 2025)
Fig.1 Sample analysis workflow. (Xie G., et al., 2025)
The limitations of liver biopsy have spurred the development of non-invasive methods for diagnosing liver fibrosis. These methods aim to provide accurate assessments without the need for invasive procedures. Non-invasive diagnostic tools can be broadly categorized into imaging techniques, such as ultrasound elastography and magnetic resonance elastography, and serum biomarker assays. While imaging techniques offer valuable information on liver stiffness, serum biomarker assays provide a more direct assessment of the biochemical changes associated with fibrosis. The FibraChek assay kit, developed by the Human Metabolomics Institute, represents a significant advancement in this area by leveraging liquid chromatography-tandem mass spectrometry (LC-MS/MS) to quantify specific metabolites in serum that are associated with liver fibrosis.
The FibraChek assay kit is designed to quantify L-tyrosine (Tyr) and taurocholic acid (TCA) in serum samples using LC-MS/MS. These metabolites have been shown to be strongly correlated with the degree of liver fibrosis, making them valuable biomarkers for non-invasive diagnosis. The kit includes calibration standards, quality controls (QCs), and internal standards (ISs) to ensure accurate and reliable results. The analytical validation of the FibraChek assay kit has demonstrated its robust performance across multiple parameters, including linearity, sensitivity, precision, accuracy, and stability.

The FibraChek assay kit exhibits a broad linear dynamic range, with a linearity range of 20–1000 μmol/L for L-tyrosine and 10.3–618 ng/ml for taurocholic acid. The limit of detection (LOD) was determined to be 2 μmol/L for Tyr and 1 ng/mL for TCA, while the limit of quantification (LOQ) was 20 μmol/L for Tyr and 10.6 ng/mL for TCA. These low LOD and LOQ values indicate that the assay can detect and quantify these metabolites even at very low concentrations, making it suitable for early-stage fibrosis detection.
The precision of the FibraChek assay was evaluated through intra-day and inter-day measurements. The intra-day precision, assessed within 24 hours, and the inter-day precision, assessed over five consecutive days, both showed acceptable variability, with coefficients of variation (CVs) less than 15%. The accuracy of the assay was further confirmed through recovery experiments, where known amounts of metabolites were spiked into serum samples. The recovery rates were between 90% and 110%, indicating high accuracy.
The clinical utility of the FibraChek assay was evaluated using serum samples from 173 liver fibrosis patients and 167 healthy controls. The results showed that both L-tyrosine and taurocholic acid levels were significantly higher in fibrosis patients compared to healthy controls. A predictive score was developed based on these metabolites, which achieved an area under the receiver operating characteristic (ROC) curve (AUC) of 0.95, with a sensitivity of 86.0% and specificity of 90.0%. This indicates that the FibraChek assay can effectively differentiate between liver fibrosis patients and healthy individuals.
The stability of the FibraChek assay kit was tested under various conditions. The kit remained stable for up to 14 months when stored at 2–8 °C, which is a significant duration that allows for long-term storage without compromising the integrity of the components. After reconstitution, the components were stable for up to 7 days at room temperature and 30 days at 2–8 °C, providing flexibility in handling and usage. The metabolites themselves were stable for up to 36 months at -20 °C or -80 °C, ensuring that the key elements of the assay can be preserved effectively over an extended period.
The FibraChek assay kit offers several advantages over traditional diagnostic methods:

The FibraChek assay kit represents a significant advancement in the non-invasive diagnosis of liver fibrosis. Its analytical performance and clinical validation suggest that it could be a valuable tool for clinicians in the early detection and management of liver fibrosis. Future studies should focus on evaluating the assay's utility in larger and more diverse patient populations to further confirm its clinical relevance. Additionally, the integration of this assay into routine clinical practice could streamline the diagnostic process and improve patient outcomes.
The FibraChek assay kit is a groundbreaking development in the field of liver fibrosis diagnosis. By leveraging advanced LC-MS/MS technology, it offers a non-invasive, accurate, and reliable method for quantifying key metabolites associated with liver fibrosis. This innovative tool has the potential to transform clinical practice, providing healthcare professionals with a powerful means to detect and manage liver fibrosis, ultimately improving patient outcomes.
If you have related needs, please feel free to contact us for more information or product support.
Reference
This article is for research use only. Do not use in any diagnostic or therapeutic application.

Cat.No. GP-DQL-00203
Rotavirus Antigen Group A and Adenovirus Antigen Rapid Test Kit (Colloidal Gold)

Cat.No. GP-DQL-00206
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Card Style

Cat.No. GP-DQL-00207
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. GP-DQL-00211
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. GP-DQL-00212
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. IP-00189
Influenza A Rapid Assay Kit

Cat.No. GH-DQL-00200
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00201
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00202
Luteinizing Hormone Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00208
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. GH-DQL-00209
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit(Colloidal Gold), Strip Style

Cat.No. GH-DQL-00210
Luteinizing Hormone Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. IH-HYW-0001
hCG Pregnancy Test Strip

Cat.No. IH-HYW-0002
hCG Pregnancy Test Cassette

Cat.No. IH-HYW-0003
hCG Pregnancy Test Midstream

Cat.No. GD-QCY-0001
Cocaine (COC) Rapid Test Kit

Cat.No. GD-QCY-0002
Marijuana (THC) Rapid Test Kit

Cat.No. GD-QCY-0003
Morphine (MOP300) Rapid Test Kit

Cat.No. GD-QCY-0004
Methamphetamine (MET) Rapid Test Kit

Cat.No. GD-QCY-0005
Methylenedioxymethamphetamine ecstasy (MDMA) Rapid Test Kit

Cat.No. GD-QCY-0006
Amphetamine (AMP) Rapid Test Kit

Cat.No. GD-QCY-0007
Barbiturates (BAR) Rapid Test Kit

Cat.No. GD-QCY-0008
Benzodiazepines (BZO) Rapid Test Kit

Cat.No. GD-QCY-0009
Methadone (MTD) Rapid Test Kit

Cat.No. GD-QCY-0011
Opiate (OPI) Rapid Test Kit

Cat.No. ID-HYW-0002
Multi-Drug Test L-Cup, (5-16 Para)

Cat.No. ID-HYW-0005
Multi-Drug Rapid Test (Dipcard & Cup) with Fentanyl

Cat.No. ID-HYW-0006
Multi-Drug Rapid Test (Dipcard & Cup) without Fentanyl

Cat.No. ID-HYW-0007
Multi-Drug 2~14 Drugs Rapid Test (Dipstick & Dipcard & Cup)

Cat.No. ID-HYW-0008
Fentanyl (FYL) Rapid Test (For Prescription Use)

Cat.No. ID-HYW-0009
Fentanyl Urine Test Cassette (CLIA Waived)

Cat.No. ID-HYW-0010
Fentanyl Urine Test Cassette (Home Use)
|
There is no product in your cart. |