- Home
- Resource
- Explore & Learn
- Breakthrough in Diagnosing Aspirin-Induced Respiratory Disease: The Aspirin Hypersensitivity Diagnostic Index
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Aspirin-induced respiratory disease (N-ERD) is a complex and often debilitating condition affecting many individuals who experience severe respiratory reactions to aspirin and other nonsteroidal anti-inflammatory drugs (NSAIDs). This condition is characterized by chronic rhinosinusitis with nasal polyps, asthma, and a significant reduction in quality of life. However, diagnosing N-ERD has long been challenging due to the lack of reliable biomarkers and the variability in clinical presentations. Recently, a groundbreaking study published in the journal Allergy introduced the Aspirin Hypersensitivity Diagnostic Index (AHDI), a novel in vitro diagnostic tool that promises to revolutionize the diagnosis of N-ERD.
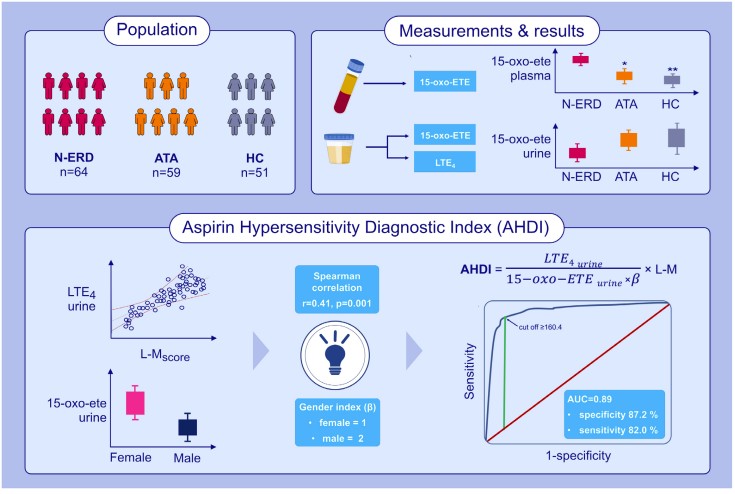 Fig.1 In vitro test for diagnosing of N-ERD based on urinary 15-oxo-ETE and LTE4 excretion. (Mastalerz L., et al., 2025)
Fig.1 In vitro test for diagnosing of N-ERD based on urinary 15-oxo-ETE and LTE4 excretion. (Mastalerz L., et al., 2025)
N-ERD is a specific asthma phenotype that manifests through acute respiratory reactions following the ingestion of NSAIDs, including aspirin. This condition is often accompanied by chronic rhinosinusitis with nasal polyps (CRSwNP), making it particularly difficult to manage and treat. The underlying pathophysiology of N-ERD involves the dysregulation of arachidonic acid (AA) metabolism, leading to the overproduction of pro-inflammatory leukotrienes and prostaglandins. One key metabolite, 15-oxo-eicosatetraenoic acid (15-oxo-ETE), has been identified as a potential biomarker for this condition.
Identifying a reliable biomarker for N-ERD has been a significant challenge for researchers and clinicians. Traditional diagnostic methods, such as oral aspirin provocation tests, are invasive and carry risks of severe reactions. Therefore, there has been a need for a non-invasive, accurate, and easily accessible diagnostic tool. The recent study by Mastalerz et al. (2025) addresses this need by proposing the AHDI, an index based on the measurement of urinary 15-oxo-ETE and leukotriene E4 (LTE4) levels.
The AHDI is a composite index that incorporates urinary LTE4-to-15-oxo-ETE ratios, corrected for sex and the severity of chronic rhinosinusitis as measured by the Lund-Mackay score. This index was developed using machine learning algorithms to provide the best distinction between N-ERD patients and those with aspirin-tolerant asthma (ATA). The study involved 64 patients with N-ERD, 59 patients with ATA, and 51 healthy controls. The results showed that the AHDI outperformed single measurements of 15-oxo-ETE or LTE4 alone, with an area under the ROC curve of 0.889, sensitivity of 81.97%, specificity of 87.23%, and accuracy of 86.87%.
The study was meticulously designed to ensure robust results. Participants underwent comprehensive clinical evaluations, including computed tomography (CT) scans of the paranasal sinuses, spirometry, and measurement of plasma and urinary levels of 15-oxo-ETE and LTE4 using high-performance liquid chromatography and tandem mass spectrometry (HPLC-MS/MS). The technical repeatability and precision of these measurements were validated, ensuring the reliability of the data.
The study revealed several key findings:
The AHDI offers several advantages over existing diagnostic methods for N-ERD:
While the AHDI represents a significant advancement in the diagnosis of N-ERD, further research is needed to fully understand its clinical utility. Future studies should focus on:
The introduction of the Aspirin Hypersensitivity Diagnostic Index (AHDI) marks a significant milestone in the diagnosis of N-ERD. By leveraging the measurement of urinary 15-oxo-ETE and LTE4, corrected for sex and the severity of chronic rhinosinusitis, the AHDI offers a reliable, non-invasive, and accurate diagnostic tool. This innovation has the potential to transform clinical practice, improve patient outcomes, and enhance the quality of life for individuals suffering from this challenging condition. As research continues to unfold, the AHDI stands as a beacon of hope for better diagnosis and management of N-ERD.
If you have related needs, please feel free to contact us for more information or product support.
Reference
This article is for research use only. Do not use in any diagnostic or therapeutic application.

Cat.No. GP-DQL-00203
Rotavirus Antigen Group A and Adenovirus Antigen Rapid Test Kit (Colloidal Gold)

Cat.No. GP-DQL-00206
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Card Style

Cat.No. GP-DQL-00207
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. GP-DQL-00211
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. GP-DQL-00212
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. IP-00189
Influenza A Rapid Assay Kit

Cat.No. GH-DQL-00200
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00201
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00202
Luteinizing Hormone Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00208
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. GH-DQL-00209
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit(Colloidal Gold), Strip Style

Cat.No. GH-DQL-00210
Luteinizing Hormone Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. IH-HYW-0001
hCG Pregnancy Test Strip

Cat.No. IH-HYW-0002
hCG Pregnancy Test Cassette

Cat.No. IH-HYW-0003
hCG Pregnancy Test Midstream

Cat.No. GD-QCY-0001
Cocaine (COC) Rapid Test Kit

Cat.No. GD-QCY-0002
Marijuana (THC) Rapid Test Kit

Cat.No. GD-QCY-0003
Morphine (MOP300) Rapid Test Kit

Cat.No. GD-QCY-0004
Methamphetamine (MET) Rapid Test Kit

Cat.No. GD-QCY-0005
Methylenedioxymethamphetamine ecstasy (MDMA) Rapid Test Kit

Cat.No. GD-QCY-0006
Amphetamine (AMP) Rapid Test Kit

Cat.No. GD-QCY-0007
Barbiturates (BAR) Rapid Test Kit

Cat.No. GD-QCY-0008
Benzodiazepines (BZO) Rapid Test Kit

Cat.No. GD-QCY-0009
Methadone (MTD) Rapid Test Kit

Cat.No. GD-QCY-0011
Opiate (OPI) Rapid Test Kit

Cat.No. ID-HYW-0002
Multi-Drug Test L-Cup, (5-16 Para)

Cat.No. ID-HYW-0005
Multi-Drug Rapid Test (Dipcard & Cup) with Fentanyl

Cat.No. ID-HYW-0006
Multi-Drug Rapid Test (Dipcard & Cup) without Fentanyl

Cat.No. ID-HYW-0007
Multi-Drug 2~14 Drugs Rapid Test (Dipstick & Dipcard & Cup)

Cat.No. ID-HYW-0008
Fentanyl (FYL) Rapid Test (For Prescription Use)

Cat.No. ID-HYW-0009
Fentanyl Urine Test Cassette (CLIA Waived)

Cat.No. ID-HYW-0010
Fentanyl Urine Test Cassette (Home Use)
|
There is no product in your cart. |