- Home
- Resource
- Disease Diagnosis
- Cancers
- Breaking Barriers in Ovarian Cancer Diagnosis: Next-Generation IVD Approaches
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Ovarian cancer remains one of the most lethal gynecological malignancies, primarily due to its frequent diagnosis at advanced stages when treatment options become limited. This comprehensive resource examines how next-generation in vitro diagnostics (IVD) are transforming ovarian cancer detection and management through advanced biomarker panels and cutting-edge technologies.
Ovarian cancer remains one of the most challenging gynecologic malignancies, characterized by its insidious onset and frequent diagnosis at advanced stages when treatment options are limited. Often called a "silent killer," its non-specific symptoms like abdominal bloating and pelvic discomfort are easily overlooked until the disease has progressed. With a high mortality-to-incidence ratio, it poses a significant global health burden, particularly affecting postmenopausal women.
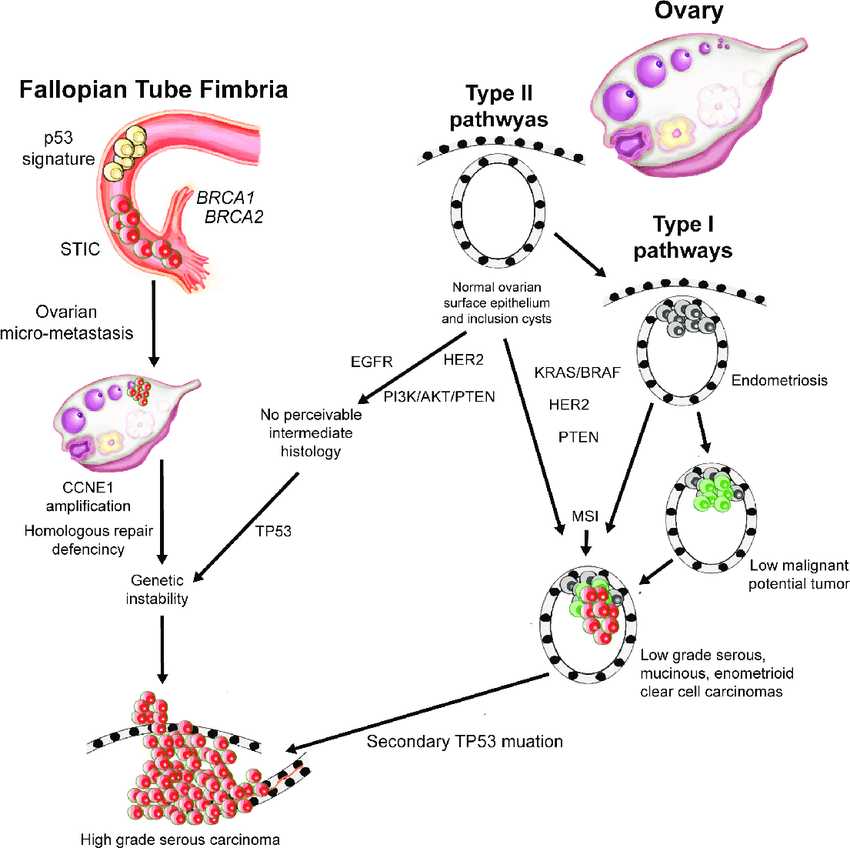 Fig.1 Pathogenesis pathways of ovarian cancer. (Hirst J, et al., 2018)
Fig.1 Pathogenesis pathways of ovarian cancer. (Hirst J, et al., 2018)

Next-generation in vitro diagnostics (IVD) are revolutionizing ovarian cancer diagnosis by overcoming the critical limitations of conventional methods through advanced multi-analyte profiling and liquid biopsy technologies. By integrating novel biomarkers like HE4 with CA-125 in sophisticated algorithms, and leveraging circulating tumor DNA (ctDNA) for mutation detection, these approaches enable significantly improved early detection accuracy and differential diagnosis of pelvic masses. This paradigm shift toward molecular-level analysis provides clinicians with powerful tools for risk stratification, timely intervention, and personalized treatment monitoring, ultimately transforming patient outcomes in this challenging disease.
The evolution of ovarian cancer diagnostics has been fundamentally shaped by the discovery and application of specific biomarkers, with the landscape expanding from single protein measurements to sophisticated multi-analyte and molecular analyses. These biomarkers provide critical, minimally invasive tools for detection, differential diagnosis, and monitoring.
CA-125
As the most established and widely used biomarker, CA-125 is a glycoprotein detected in serum, primarily applied for monitoring treatment response and detecting recurrence in known ovarian cancer patients. However, its utility in early detection is limited by poor sensitivity for early-stage disease and low specificity, as levels can be elevated in various benign conditions such as endometriosis, pelvic inflammatory disease, and even during menstruation.
Human Epididymis Protein 4 (HE4)
This newer serum glycoprotein biomarker demonstrates superior specificity compared to CA-125 in distinguishing ovarian cancer from benign pelvic masses. HE4 is particularly valuable when combined with CA-125 in validated algorithms like the Risk of Ovarian Malignancy Algorithm (ROMA), which integrates these biomarker levels with menopausal status to provide a more accurate risk assessment for women presenting with an adnexal mass.
Circulating Tumor DNA (ctDNA)
Representing the cutting edge of liquid biopsy, ctDNA analysis involves detecting tumor-derived genetic material in the bloodstream. This approach can identify characteristic mutations (e.g., in TP53), epigenetic changes, and copy number variations, offering potential for early-stage detection, monitoring of minimal residual disease after treatment, and real-time assessment of therapeutic efficacy and emerging resistance mechanisms.
The advancement of ovarian cancer diagnostics is being propelled by a new generation of in vitro diagnostic (IVD) technologies that enable more precise, multiplexed, and sensitive biomarker analysis. These platforms are crucial for translating complex biological signatures into clinically actionable information.
Modern immunoassays have evolved into highly automated and multiplexed platforms, such as chemiluminescent microparticle immunoassays, which allow for the simultaneous quantitative measurement of multiple protein biomarkers like CA-125 and HE4 from a single serum sample. This simultaneous measurement is fundamental for calculating integrated risk assessment scores like the ROMA index, providing a more robust clinical picture than single-marker tests and enhancing the differential diagnosis of pelvic masses.

Mass spectrometry-based proteomics is emerging as a powerful discovery and validation tool for identifying novel protein biomarker panels. By precisely quantifying hundreds to thousands of proteins in a single serum sample, this technology can uncover distinctive proteomic signatures of ovarian cancer that extend far beyond traditional markers, holding significant promise for the development of highly sensitive and specific multi-marker tests for early-stage detection.

This category encompasses technologies like next-generation sequencing (NGS) and digital PCR (dPCR), which are revolutionizing liquid biopsy applications by analyzing circulating tumor DNA (ctDNA). These techniques can detect minute quantities of tumor-specific mutations (e.g., in TP53 or BRCA genes) and epigenetic alterations with exceptional sensitivity, enabling applications in early detection, monitoring minimal residual disease, and guiding targeted therapy decisions with unprecedented precision.
Alta DiagnoTech provides a comprehensive portfolio of in vitro diagnostic (IVD) solutions for ovarian cancer, supporting early detection, differential diagnosis, and personalized treatment monitoring. Our innovative products leverage cutting-edge technologies to deliver reliable, clinically actionable results that enhance patient management throughout the care continuum. If you have related needs, please feel free to contact us for more information or product support.
| Product Name | Technology | Application |
| CA-125 Quantitative Assay | Chemiluminescent Immunoassay (CLIA) | Detection and quantification of CA-125 levels in serum for disease monitoring and treatment response assessment |
| HE4 ELISA Kit | ELISA | Measurement of human epididymis protein 4 (HE4) levels in serum for differential diagnosis of pelvic masses |
| Liquid Biopsy NGS Panel for Ovarian Cancer | Next-Generation Sequencing (NGS) | Comprehensive genomic profiling of circulating tumor DNA for mutation detection and therapy guidance |
| miRNA Signature Test for Ovarian Cancer | Quantitative PCR (qPCR) | Analysis of specific microRNA signatures in serum for early detection and risk stratification |
Reference
This article is for research use only. Do not use in any diagnostic or therapeutic application.
|
There is no product in your cart. |