- Home
- Resource
- Disease Diagnosis
- Cancers
- Biomarker-Driven Management of Gastric Cancer: HER2, MSI, and PD-L1 Testing
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Gastric cancer is a complex and molecularly heterogeneous disease where precise biomarker identification is crucial for effective treatment selection. This resource provides a comprehensive guide to the essential in vitro diagnostic (IVD) tools and methodologies for accurate detection of key biomarkers, including HER2, MSI/dMMR, and PD-L1, that are fundamental for guiding targeted therapy and immunotherapy decisions in modern clinical practice.
Gastric cancer, also commonly referred to as stomach cancer, is a malignant tumor arising from the lining of the stomach. While its global incidence varies geographically, it remains a significant cause of cancer-related mortality. The majority of cases are adenocarcinomas, which are categorized into intestinal and diffuse types, each with distinct pathological and clinical features. Historically, diagnosis relied primarily on histopathological examination; however, modern management is now fundamentally guided by the identification of specific molecular biomarkers that predict response to targeted and immunotherapies, enabling a more personalized treatment approach.
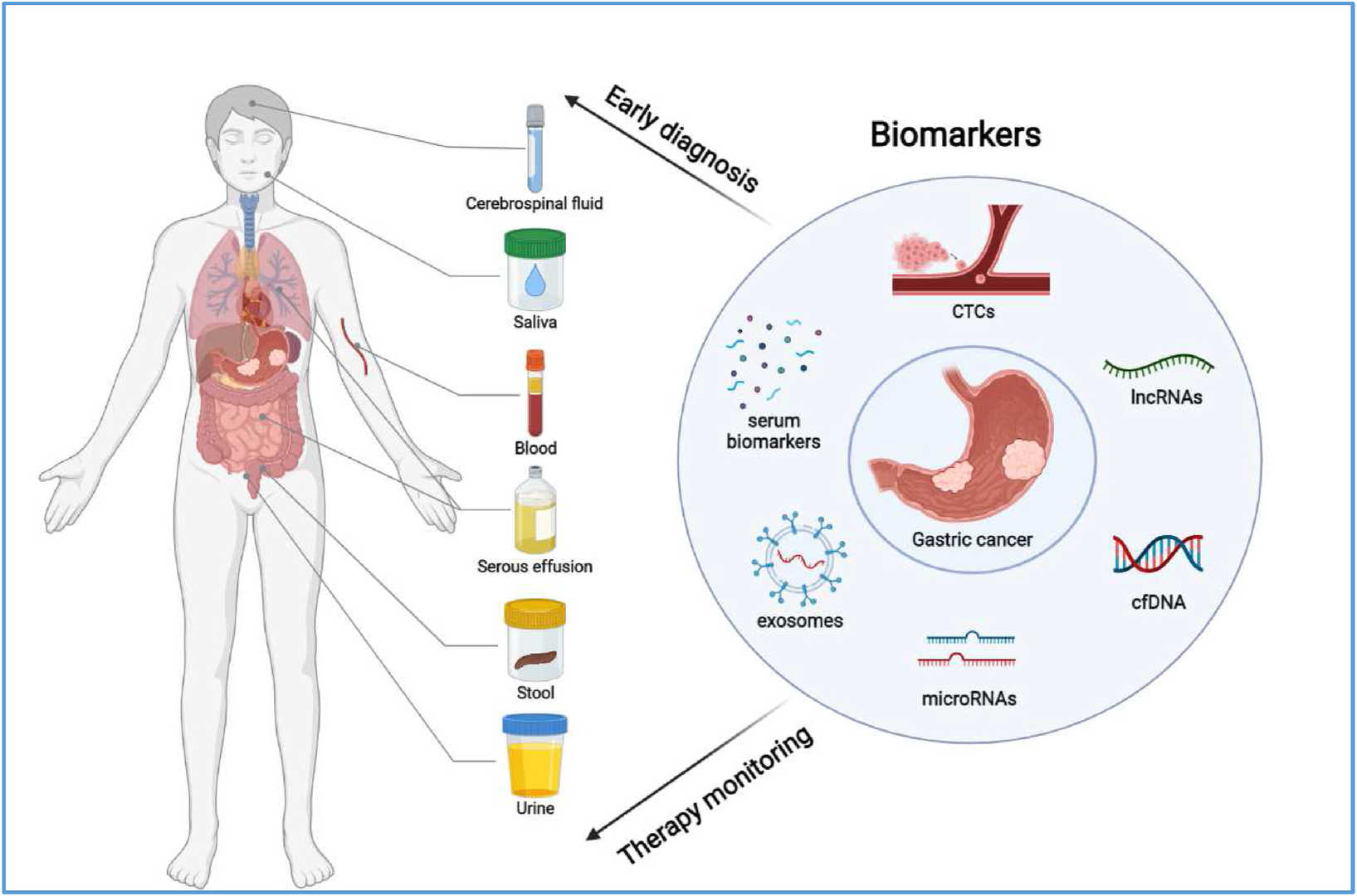 Fig.1 Scheme of biomarkers associated with gastric cancer. (Jiang T, et al., 2022)
Fig.1 Scheme of biomarkers associated with gastric cancer. (Jiang T, et al., 2022)
The definitive diagnosis of gastric cancer hinges on the histopathological examination of tissue obtained through endoscopic biopsy. This microscopic analysis of hematoxylin and eosin (H&E) stained slides confirms malignancy, classifies the tumor type—most commonly adenocarcinoma—and further subclasses it into intestinal or diffuse subtypes according to the Lauren classification. The pathologist also assesses critical prognostic features such as tumor grade, depth of invasion, and the presence of lymphovascular invasion, providing the essential foundational data upon which all subsequent staging and biomarker testing is built.
Human epidermal growth factor receptor 2 (HER2) is a well-established biomarker that has transformed the treatment landscape for a specific subset of gastric cancer patients. Accurate testing is not merely a diagnostic step but a critical gateway to effective, personalized therapy, underscoring the vital role of precise immunohistochemistry (IHC) and in situ hybridization (ISH) assays in the modern pathology laboratory.

Clinical Significance
HER2 is a critical predictive biomarker in gastric cancer. Approximately 10-20% of gastric adenocarcinomas, particularly those of the gastroesophageal junction and intestinal subtype, demonstrate HER2 protein overexpression or gene amplification. A positive HER2 test result identifies patients who are eligible for targeted therapy with anti-HER2 agents, such as trastuzumab, which, when combined with chemotherapy, significantly improves survival outcomes in advanced metastatic disease.
Testing Methodologies
Testing follows a standardized algorithm. Immunohistochemistry (IHC) is the primary method, scoring protein expression on a scale of 0 to 3+. Tumors with IHC 3+ are considered positive, while those with IHC 0 or 1+ are negative. In situ hybridization (ISH), which detects gene amplification, is used as a reflex test for equivocal IHC 2+ cases. A combined approach ensures accurate patient selection for targeted therapy.
Microsatellite instability (MSI) and mismatch repair deficiency (dMMR) represent a critical molecular signature in gastric cancer that has profound implications for treatment selection. This biomarker identifies a distinct subgroup of tumors with unique biological behavior and exceptional responsiveness to immunotherapy, making its detection a standard component of the modern diagnostic workup.

MSI/dMMR is a key predictive biomarker for immunotherapy. Present in about 10-20% of early gastric cancers and a smaller percentage of advanced cases, dMMR/MSI-H status identifies a distinct tumor subtype with a high mutational burden. These tumors are highly responsive to immune checkpoint inhibitors (e.g., anti-PD-1/PD-L1 agents), making testing essential to identify patients who can derive exceptional, durable benefit from this treatment modality.
Two complementary methods are used. Immunohistochemistry (IHC) screens for dMMR by detecting the loss of nuclear expression of the four mismatch repair proteins: MLH1, MSH2, MSH6, and PMS2. Alternatively, polymerase chain reaction (PCR) or next-generation sequencing (NGS) can be used to directly assess for MSI by comparing the length of microsatellite sequences in tumor versus normal DNA. IHC is widely used as an efficient and cost-effective first-line screening tool.
Programmed death-ligand 1 (PD-L1) testing has emerged as an important biomarker for guiding immunotherapy decisions in gastric cancer. As a key ligand in the immune checkpoint pathway, PD-L1 expression helps identify patients who are more likely to respond to immune checkpoint inhibitors, though its role as a predictive biomarker is more nuanced than other markers like MSI/dMMR.

Clinical Significance
PD-L1 testing serves as a predictive, though complementary, biomarker for immunotherapy in gastric cancer. It measures the expression of the PD-L1 protein on tumor and immune cells, which is a mechanism tumors use to suppress the immune system. The result, reported as a combined positive score (CPS), helps estimate the likelihood of response to anti-PD-1 therapies. While not an absolute requirement for all immunotherapies, a higher CPS is associated with an increased probability of treatment benefit in certain clinical settings.
Testing Methodologies
Immunohistochemistry (IHC) is the sole methodology for PD-L1 testing. The result is quantified using the combined positive score (CPS), which calculates the number of PD-L1 staining cells (tumor cells, lymphocytes, macrophages) relative to the total number of viable tumor cells. It is critical to use analytically and clinically validated IHC assays, as scoring algorithms and antibody clones can be specific to different therapeutic agents.
Alta DiagnoTech provides a comprehensive portfolio of in vitro diagnostic (IVD) solutions for gastric cancer, enabling precise biomarker detection and personalized treatment strategies. Our innovative products cover the entire diagnostic workflow, from initial histopathological analysis to advanced molecular testing, ensuring accurate detection of key biomarkers including HER2, MSI/dMMR, and PD-L1. If you have related needs, please feel free to contact us for more information or product support.
| Product Name | Technology | Application |
| HER2 IHC Complete Detection Kit | Immunohistochemistry (IHC) | Detection of HER2 protein overexpression in gastric cancer tissue specimens |
| HER2 FISH Testing System | Fluorescence In Situ Hybridization (FISH) | Confirmation of HER2 gene amplification in equivocal IHC cases |
| dMMR Panel IHC Kit | Immunohistochemistry (IHC) | Simultaneous detection of four mismatch repair proteins (MLH1, MSH2, MSH6, PMS2) |
| MSI Analysis System | PCR Fragment Analysis | Molecular detection of microsatellite instability in gastric tumor tissue |
| PD-L1 IHC Assay (22C3) | Immunohistochemistry (IHC) | PD-L1 expression analysis using Combined Positive Score (CPS) for immunotherapy guidance |
| PD-L1 IHC Assay (SP142) | Immunohistochemistry (IHC) | Alternative PD-L1 assessment for specific immunotherapy treatment options |
| Comprehensive Gastric Cancer NGS Panel | Next-Generation Sequencing | Simultaneous analysis of multiple biomarkers including HER2, MSI, and other relevant mutations |
| Automated Tissue Processing System | Automated Staining | Standardized processing of gastric tissue specimens for consistent IHC results |
Reference
This article is for research use only. Do not use in any diagnostic or therapeutic application.
|
There is no product in your cart. |