Beyond the Ultrasound: A Multimodal Imaging and Genetic Approach to Hypertrophic Cardiomyopathy (HCM) Diagnosis
Hypertrophic cardiomyopathy (HCM) is a complex genetic heart disorder characterized by unexplained thickening of the heart muscle, posing significant challenges in diagnosis and risk stratification. This resource provides a comprehensive guide to the modern, multimodal approach essential for its evaluation, moving beyond foundational ultrasound to detail the critical roles of advanced cardiac MRI for tissue characterization and genetic testing for definitive diagnosis and family screening.
Introduction to Hypertrophic Cardiomyopathy (HCM)
Hypertrophic cardiomyopathy (HCM) is a common inherited cardiovascular disease characterized by abnormal thickening of the heart muscle without an identifiable cause, such as high blood pressure. This primary hypertrophy is often asymmetric and can lead to debilitating symptoms, including shortness of breath, chest pain, and palpitations. The pathophysiology involves a complex interplay of left ventricular outflow tract obstruction, diastolic dysfunction, mitral regurgitation, and an increased risk of life-threatening arrhythmias. As the most frequent cause of sudden cardiac death in young people and athletes, its timely and accurate diagnosis is critical for effective management and family screening.
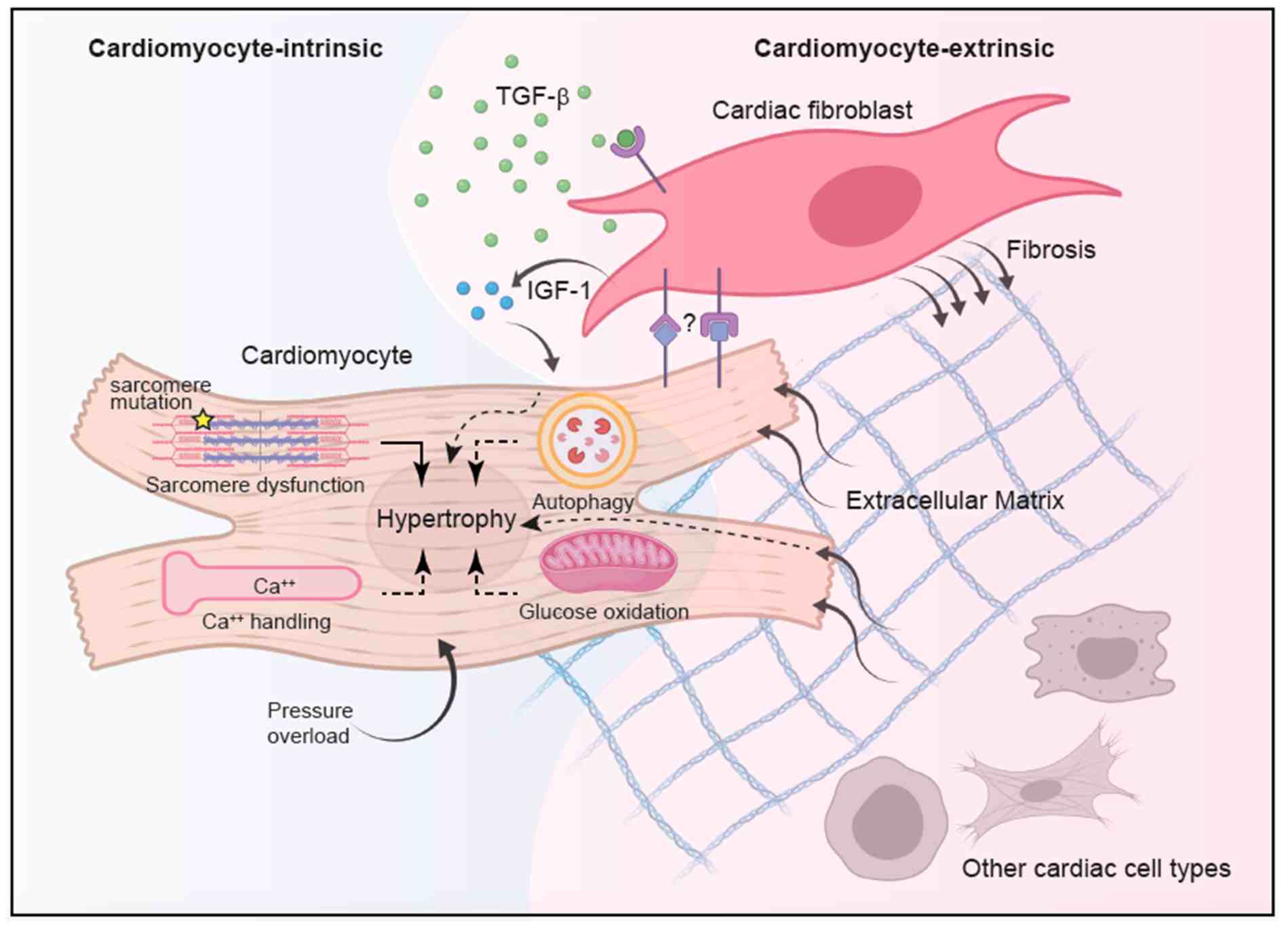 Fig.1 Integrated model of hypertrophic cardiomyopathy (HCM) pathogenesis. (Chou C, Chin M T., 2021)
Fig.1 Integrated model of hypertrophic cardiomyopathy (HCM) pathogenesis. (Chou C, Chin M T., 2021)
The Foundation: Echocardiography in Hypertrophic Cardiomyopathy (HCM)
Transthoracic echocardiography serves as the fundamental, first-line imaging modality for the diagnosis and initial assessment of hypertrophic cardiomyopathy (HCM), providing a comprehensive real-time evaluation of cardiac structure and function. Its primary role is to identify and quantify the characteristic left ventricular hypertrophy—not explained by secondary causes—and to define its distribution, such as asymmetric septal thickening. Crucially, echocardiography also dynamically assesses for left ventricular outflow tract obstruction, detects systolic anterior motion of the mitral valve, and evaluates diastolic function, establishing itself as an indispensable tool for both initial diagnosis and guiding subsequent management decisions.
The Game Changer: Cardiac Magnetic Resonance (CMR)
Cardiac magnetic resonance (CMR) has revolutionized the diagnosis and management of hypertrophic cardiomyopathy (HCM) by providing insights far beyond the capabilities of standard ultrasound. Its value lies in several key areas:
Unmatched Anatomic Precision
CMR provides high-resolution, three-dimensional images of the heart, allowing for accurate measurement of hypertrophy in challenging areas like the apex and lateral wall, ensuring no form of HCM is missed.
Tissue Characterization (LGE)
A core strength of CMR is its ability to detect and quantify myocardial fibrosis (scarring) using late gadolinium enhancement (LGE). The presence and extent of LGE are powerful, independent risk factors for sudden cardiac death.
Detecting Diffuse Disease (T1 Mapping)
Advanced techniques like T1 mapping can identify diffuse, microscopic fibrosis that is invisible to conventional LGE imaging, providing an even earlier and more comprehensive assessment of myocardial tissue health.
Differential Diagnosis
CMR is crucial for distinguishing HCM from other conditions that cause heart thickening (phenocopies), such as cardiac amyloidosis and Fabry disease, through their unique tissue signature on parametric maps.
The Blueprint: Genetic Testing in Hypertrophic Cardiomyopathy (HCM)
Genetic testing in hypertrophic cardiomyopathy (HCM) is far more than a diagnostic tool; it is the foundational blueprint that reveals the underlying molecular cause of the disease. As the majority of HCM cases are caused by pathogenic variants in sarcomere protein genes, genetic analysis provides a definitive explanation for the clinical phenotype. This shifts the management paradigm from being solely symptom-based to incorporating a proactive, etiology-driven strategy. Understanding the genetic basis is crucial not only for confirming the diagnosis in the proband but also for enabling precise risk assessment and targeted family screening, ultimately paving the way for personalized medicine. Key applications of genetic testing in HCM include:
Confirming the Diagnosis and Establishing Etiology
Genetic testing can provide a definitive diagnosis in patients with borderline or atypical clinical features. A positive result confirms the disease is inherited and rooted in a sarcomeric mutation, distinguishing it from physiological hypertrophy or other phenocopies.
Guiding Family Screening (Cascade Testing)
This is one of the most critical applications. Once a pathogenic variant is identified in the proband, targeted genetic testing can be offered to all first-degree relatives. This allows for the precise identification of at-risk family members who have inherited the mutation (genotype-positive), enabling early monitoring and intervention, even before clinical signs develop.
Informing Prognosis and Risk Stratification
Although still evolving, specific gene mutations (e.g., in MYH7, MYBPC3, TNNT2) are associated with different risks of adverse outcomes. While genotype should be integrated with clinical markers, it can contribute to a more personalized assessment of sudden cardiac death risk.
Aiding in Differential Diagnosis
Genetic testing helps distinguish classic sarcomeric HCM from other conditions that mimic its presentation, known as phenocopies (e.g., cardiac amyloidosis, Fabry disease, Danon disease). Identifying a non-sarcomeric mutation directs patients towards the correct, often disease-specific, management pathway.
Featured Products for HypertrophicCardiomyopathy (HCM) Diagnostics
As a leading provider of cardiovascular disease diagnostic solutions, Alta DiagnoTech delivers an integrated portfolio of both in vitro diagnostics (IVD) and research-use-only (RUO) solutions for hypertrophic cardiomyopathy (HCM). Our dual approach bridges clinical practice with scientific discovery, offering robust diagnostic tools for accurate patient assessment alongside cutting-edge research products that enable breakthrough discoveries in HCM pathogenesis and treatment. If you have related needs, please feel free to contact us for more information or product support.
| Product Name |
Technology |
Application |
| High-Sensitivity Cardiac Troponin I Assay |
Chemiluminescence Immunoassay (CLIA) |
Quantitative measurement of cardiac troponin I in serum/plasma for detection of myocardial injury in HCM; IVD |
| NT-proBNP Electrochemiluminescence Assay |
Electrochemiluminescence Immunoassay (ECLIA) |
Quantitative measurement of NT-proBNP in serum/plasma to aid in heart failure evaluation in HCM patients; IVD |
| HCM Comprehensive Genetic Panel |
Next-Generation Sequencing (NGS) |
Detection of pathogenic variants in core sarcomere and HCM-associated genes (MYBPC3, MYH7, TNNI3, etc.) for diagnostic confirmation and family screening; RUO |
| sST2 ELISA Kit |
Enzyme-Linked Immunosorbent Assay (ELISA) |
Quantitative detection of soluble ST2 in human serum for research on myocardial fibrosis and remodeling in HCM; RUO |
| Galectin-3 CLIA Kit |
Chemiluminescence Immunoassay (CLIA) |
Quantitative measurement of Galectin-3 levels in plasma/serum for research on cardiac fibrosis and prognosis; RUO |
| Cardiac Myosin-Binding Protein C (cMyBP-C) ELISA |
Enzyme-Linked Immunosorbent Assay (ELISA) |
Quantitative analysis of cMyBP-C in serum as a potential research biomarker for sarcomeric dysfunction; RUO |
Reference
- Chou C, Chin M T. Pathogenic mechanisms of hypertrophic cardiomyopathy beyond sarcomere dysfunction[J]. International journal of molecular sciences, 2021, 22(16): 8933.
This article is for research use only. Do not use in any diagnostic or therapeutic application.



 Fig.1 Integrated model of hypertrophic cardiomyopathy (HCM) pathogenesis. (Chou C, Chin M T., 2021)
Fig.1 Integrated model of hypertrophic cardiomyopathy (HCM) pathogenesis. (Chou C, Chin M T., 2021)