- Home
- Resource
- Disease Diagnosis
- Cancers
- Beyond the Tumor: Leveraging Liquid Biomarkers in Neuroblastoma Diagnosis
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Neuroblastoma is a complex embryonic tumor of the peripheral nervous system, representing the most common extracranial solid cancer in childhood. This resource provides a comprehensive overview of the modern diagnostic landscape for neuroblastoma, detailing the integrated clinical pathway from initial suspicion through radiological and histological confirmation to molecular profiling. The following sections will systematically explore the essential roles of imaging modalities, tissue biopsy, and the growing importance of liquid biomarkers in risk stratification, treatment monitoring, and disease management.
Neuroblastoma is an embryonic cancer of the peripheral nervous system, representing the most common extracranial solid tumor in infants and young children. It arises from neural crest cells and demonstrates remarkably heterogeneous clinical behavior, ranging from spontaneous regression to aggressive, metastatic disease. Diagnosis relies on a combination of imaging studies, histopathological confirmation from a tissue biopsy, and the detection of specific tumor biomarkers, with risk stratification and treatment strategies heavily dependent on age, disease stage, and molecular characteristics such as MYCN amplification.
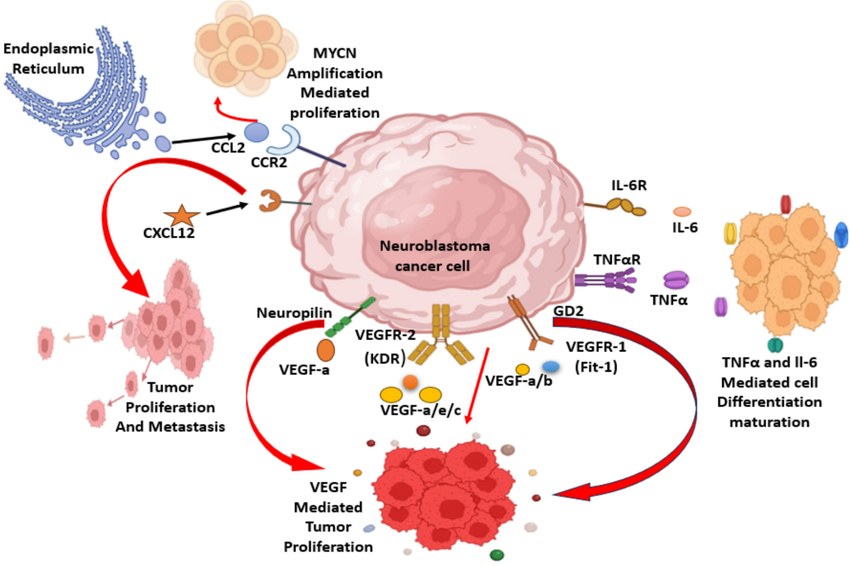 Fig.1 The pathogenesis of neuroblastoma progression and various biomarkers involved in tumor proliferation. (Rajpoot S, et al., 2025)
Fig.1 The pathogenesis of neuroblastoma progression and various biomarkers involved in tumor proliferation. (Rajpoot S, et al., 2025)
The definitive diagnosis and staging of neuroblastoma rely on a multimodal approach that directly visualizes the tumor and obtains tissue for pathological analysis. These foundational tools are critical for confirming the disease, determining its extent, and acquiring the necessary samples for further molecular testing, which collectively guide all subsequent treatment decisions.

Imaging is essential for locating the primary tumor, typically found in the abdomen or chest, and for detecting metastatic spread. Key techniques include ultrasound and CT/MRI for initial anatomical assessment, while the meta-iodobenzylguanidine (MIBG) scan serves as a highly specific functional imaging study that actively seeks out neuroblastoma cells throughout the body, making it indispensable for accurate staging.

A tissue biopsy, obtained either surgically or via needle core, provides the definitive diagnosis. Histopathological examination confirms the presence of a small round blue cell tumor, and this tissue sample is irreplaceable for performing critical molecular analyses, such as testing for MYCN gene amplification and DNA ploidy, which are paramount for risk stratification.
Liquid biopsy is an emerging diagnostic approach that analyzes tumor-derived biomarkers from biofluids, primarily blood. This minimally invasive technique provides a dynamic window into tumor biology, offering significant advantages over traditional tissue biopsy. Its core value lies in capturing tumor heterogeneity and enabling real-time monitoring, which is particularly valuable in a disease as clinically variable as neuroblastoma.
Key Advantages
Core Applications
Liquid biomarkers have revolutionized neuroblastoma diagnostics by providing minimally invasive tools for detection, risk stratification, and disease monitoring. These serum and molecular biomarkers, detectable through blood tests, offer real-time insights into tumor biology and treatment response, enabling more personalized patient management.
Alta DiagnoTech provides a comprehensive portfolio of advanced IVD products for neuroblastoma diagnostics, delivering precise tools for molecular profiling and disease monitoring. Our ready-to-use reagent kits and integrated systems provide healthcare professionals with standardized, reproducible solutions for obtaining critical diagnostic and prognostic data, supporting personalized treatment decisions throughout the patient care continuum. If you have related needs, please feel free to contact us for more information or product support.
| Product Name | Technology | Application |
| Urinary Catecholamine Metabolites (HVA/VMA) Assay | High-Performance Liquid Chromatography (HPLC) | Initial diagnosis and disease activity monitoring |
| MYCN Amplification Detection Assay | Next-Generation Sequencing (NGS) | Risk stratification and prognosis assessment |
| ALK Mutation Analysis Panel | Digital PCR / NGS | Targeted therapy selection and treatment guidance |
| Circulating Tumor DNA (ctDNA) Monitoring Panel | Next-Generation Sequencing (NGS) | Minimal residual disease detection and therapy response monitoring |
| Serum LDH Quantification Assay | Enzymatic Immunoassay | Tumor burden assessment and treatment response evaluation |
| Neuron-Specific Enolase (NSE) Detection Kit | Chemiluminescent Immunoassay (CLIA) | Disease progression monitoring and relapse detection |
| DNA Ploidy Analysis Assay | Flow Cytometry | Prognostic evaluation and risk classification |
Reference
This article is for research use only. Do not use in any diagnostic or therapeutic application.
|
There is no product in your cart. |