Devices
In the evolving world of healthcare, the role of medical devices is more critical than ever. From simple diagnostic tools to sophisticated AI-driven technologies, medical devices are at the heart of effective treatment, prevention, and rehabilitation. Over the years, the U.S. Food and Drug Administration (FDA) has been responsible for ensuring that these devices meet stringent safety and efficacy standards. Among the various regulatory pathways available, the 510(k) process has long been the dominant approach for moderate-risk devices, making up 99% of all FDA-regulated devices. However, the rise of novel technologies, particularly software-based devices, has led to the creation of an alternative route—the De Novo pathway.
The De Novo classification process was established to allow novel medical devices that lack a suitable predicate device to be approved under an expedited regulatory framework. This process not only facilitates the entry of innovative technologies into the market but also allows the FDA to establish new categories for devices, providing a critical step toward modernizing healthcare technology.
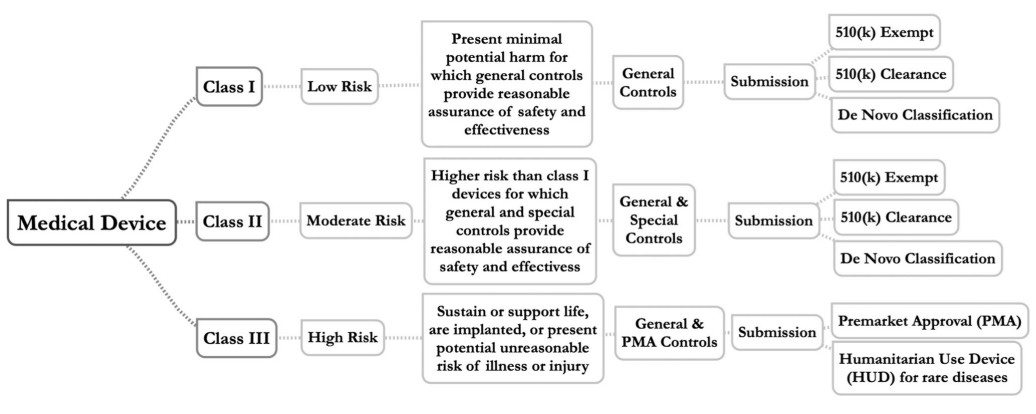 Fig.1 Device risk classification, regulatory controls, and regulatory submission pathways. (Aboy M., et al., 2024)
Fig.1 Device risk classification, regulatory controls, and regulatory submission pathways. (Aboy M., et al., 2024)
The Regulatory Landscape of Medical Devices
Classifications and Pathways
In the U.S., medical devices are classified into three categories based on risk:
- Class I: Low-risk devices that are subject to general controls.
- Class II: Moderate-risk devices that are subject to both general and special controls.
- Class III: High-risk devices that require more stringent controls, often involving premarket approval (PMA).
Traditionally, the 510(k) pathway has been the primary method for most devices to gain FDA approval. This pathway allows a device to be marketed by demonstrating substantial equivalence to an existing predicate device. For moderate-risk devices, the 510(k) process has been widely used, particularly in the case of diagnostic tools and simple medical equipment. However, as healthcare technology advanced, especially with the rise of software as a medical device (SaMD) and AI-enabled technologies, the limitations of the 510(k) pathway became increasingly apparent.
The Need for the De Novo Pathway
The De Novo pathway, introduced in the FDA Modernization Act of 1997, was specifically designed to address this gap. It allows manufacturers to introduce new devices that do not have a legally marketed predicate device, providing a route for novel technologies that don't fit into the existing regulatory categories. Devices approved through the De Novo process are classified into Class I or Class II, based on their risk profile, and set a precedent for future devices in the same category.
This pathway is particularly crucial for the approval of devices involving cutting-edge technologies, including diagnostic tools, wearables, and mobile health applications. The De Novo process has significantly supported the rise of software-based devices, such as mobile health applications and AI-powered diagnostic tools, which cannot always be compared to traditional hardware devices.
How the De Novo Pathway Supports Innovation
Facilitating Market Entry for Novel Devices

The core advantage of the De Novo pathway lies in its ability to facilitate the market entry of novel devices that do not have a predicate. Under the 510(k) process, the requirement for substantial equivalence often places constraints on device manufacturers, especially those working on innovative technologies. If no predicate device exists, the manufacturer could be forced to pursue a Premarket Approval (PMA) application, a much more rigorous and time-consuming process reserved for high-risk devices.
In contrast, the De Novo process streamlines the approval process for new technologies. Once a device is classified through De Novo, it establishes a new product code and regulatory category, which can then be used as a predicate for future submissions. This aspect of the process not only enables manufacturers to bring their novel devices to market faster but also creates opportunities for future innovations to build upon the newly established device category.
Impact on Software as a Medical Device (SaMD)
One of the most significant areas where the De Novo pathway has made an impact is in the field of Software as a Medical Device (SaMD). SaMD refers to software that is intended for medical purposes but does not require hardware to perform its intended function. Examples include mobile health apps, AI-based diagnostic tools, and software that monitors and analyzes patient data.
As the healthcare industry increasingly turns to digital solutions, SaMD devices are becoming integral in diagnosis, monitoring, and personalized healthcare. For instance, the Apple ECG App, classified under the De Novo pathway, established a new category for over-the-counter photoplethysmograph analysis software. This innovation not only demonstrated the viability of SaMD but also showcased the power of the De Novo pathway in supporting digital health technologies.
The global market for SaMDs is projected to grow significantly, with the market value expected to reach $86.45 billion by 2027. This growth is fueled by advances in mobile technology, wearables, and the increasing demand for remote healthcare solutions, particularly in the context of global health crises like the COVID-19 pandemic. The De Novo pathway is thus becoming an essential tool for the approval and adoption of innovative digital health solutions.
Competition and Intellectual Property (IP) Considerations
Encouraging Market Competition

The De Novo pathway also has significant implications for market competition. While the 510(k) process allows for quicker market entry based on the concept of substantial equivalence, it does not necessarily encourage innovation. In contrast, the De Novo process promotes first-mover advantages, where the first device in a new category sets the stage for future innovations.
Once a device has been classified via the De Novo process, it becomes the first predicate for similar devices, allowing follow-on devices to be cleared through the 510(k) pathway. This dynamic can both lower and raise barriers to market entry. On the one hand, new entrants can leverage the De Novo-approved device as a predicate, which makes it easier and faster to bring similar devices to market. On the other hand, first movers can influence the regulatory requirements for future competitors, particularly in the case of complex devices that involve clinical studies or specific performance standards.
For example, Apple's successful De Novo classification of the ECG App required the company to conduct a pivotal clinical study. This clinical data not only facilitated the device's market entry but also established new performance standards for future devices in the same category. As a result, competitors seeking to enter the market must now adhere to similar clinical testing requirements, which may raise the cost and complexity of entry.
The Role of Intellectual Property (IP)

Intellectual property plays a pivotal role in the De Novo process, particularly for innovative devices. Once a device has been approved via De Novo, the patent associated with its core technology can create a competitive advantage for the manufacturer. Moreover, the establishment of a new device category often leads to the patenting of new technologies and proprietary algorithms, especially in the case of software-based medical devices.
For SaMD, the patenting of AI algorithms and data-driven technologies has become increasingly important. The first mover in a new device category can set high performance standards that later entrants must meet. This is especially significant in the case of machine learning algorithms, where the initial dataset used to train the algorithm may give the first device a competitive edge.
The De Novo pathway, combined with strong IP protections, can thus help manufacturers secure long-term market dominance by controlling key technologies and setting performance benchmarks that competitors must meet.
Future Trends and the Role of De Novo in Medical Device Innovation
The Growing Importance of AI and Machine Learning
As medical devices become increasingly reliant on AI and machine learning (ML), the role of the De Novo pathway will continue to grow. Traditional regulatory processes like 510(k) struggle to address the complexities of AI-powered devices, which often introduce novel algorithms and machine learning models that cannot easily be compared to existing products.
The FDA's De Novo pathway has already been instrumental in classifying AI-driven devices, such as AI-based diagnostic tools and predictive health monitoring systems. These devices rely on algorithms that process large datasets to make predictions about patient health, detect diseases early, or recommend treatment plans. Because AI devices evolve rapidly, it is essential to have a regulatory pathway that can accommodate these innovations without delaying their market entry.
In the future, as AI technologies continue to advance, the De Novo pathway will likely be the go-to route for many AI-enabled medical devices. The ability to classify these devices quickly and create new product categories will be critical to ensuring that healthcare systems can benefit from the latest advancements.
Updating and Modernizing Regulatory Categories
One of the key functions of the De Novo pathway is its ability to update and modernize existing medical device categories. As healthcare technologies evolve, there is a growing need to adapt the regulatory framework to reflect the latest innovations. The De Novo process provides the FDA with a mechanism to create new product codes and regulatory classifications for devices that were previously unclassified or underserved by the 510(k) process.
The increasing reliance on software and AI in medical devices underscores the need for an agile regulatory system that can keep pace with technological advances. The De Novo pathway, by creating new categories and predicates, is helping to fill this gap, ensuring that the FDA remains responsive to emerging trends in healthcare technology.
Conclusion
The FDA's De Novo classification pathway represents a key innovation enabler in the rapidly evolving field of medical devices. By allowing manufacturers to introduce novel technologies that do not have a suitable predicate device, the De Novo process fosters innovation, supports the development of software-based devices, and encourages the creation of new medical device categories.
As the medical device industry continues to embrace digital health, AI, and machine learning technologies, the De Novo pathway will play an increasingly vital role in ensuring that healthcare systems have access to the latest innovations. By promoting both competition and intellectual property protections, the De Novo process provides a dynamic framework for regulatory flexibility and future-proofing healthcare technologies.
In the future, we can expect the De Novo pathway to continue to be a catalyst for innovation, helping to unlock new possibilities in healthcare and setting the stage for the next generation of medical devices.
If you have related needs, please feel free to contact us for more information or product support.
Reference
- Aboy, Mateo, Cristina Crespo, and Ariel Stern. "Beyond the 510 (k): The regulation of novel moderate-risk medical devices, intellectual property considerations, and innovation incentives in the FDA's De Novo pathway." NPJ Digital Medicine 7.1 (2024): 29.
This article is for research use only. Do not use in any diagnostic or therapeutic application.
Trending Products



 Fig.1 Device risk classification, regulatory controls, and regulatory submission pathways. (Aboy M., et al., 2024)
Fig.1 Device risk classification, regulatory controls, and regulatory submission pathways. (Aboy M., et al., 2024) 



































