- Home
- Resource
- Disease Diagnosis
- Endocrine Diseases
- Beyond Low Calcium: The Essential Biomarker Workflow for Hypoparathyroidism
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Hypoparathyroidism is an endocrine disorder defined by deficient parathyroid hormone (PTH) production, leading to hypocalcemia, hyperphosphatemia, and a range of clinical symptoms. This resource provides a systematic guide to its laboratory diagnosis, moving beyond the initial finding of low calcium to outline the essential biomarker workflow. We will detail the step-by-step process to confirm the biochemical signature, characterize the metabolic sequelae, and differentiate between underlying causes—concluding with an overview of the specialized in vitro diagnostic (IVD) tools that enable precise and efficient evaluation at each stage.
Hypoparathyroidism is an endocrine disorder characterized by inadequate production or secretion of parathyroid hormone (PTH) from the parathyroid glands, leading to a distinct biochemical triad of hypocalcemia, hyperphosphatemia, and inappropriately low or undetectable PTH levels. Most commonly resulting from accidental damage or removal of the glands during neck surgery, it can also arise from autoimmune destruction, genetic defects, or idiopathic causes. This PTH deficiency disrupts normal mineral homeostasis, impairing bone resorption, renal calcium reabsorption, and vitamin D activation, which may result in symptoms ranging from neuromuscular irritability (e.g., paresthesia, cramping, tetany) to long-term complications affecting the kidneys, brain, and bones. Accurate diagnosis relies on recognizing this characteristic biochemical signature and differentiating it from other causes of hypocalcemia.
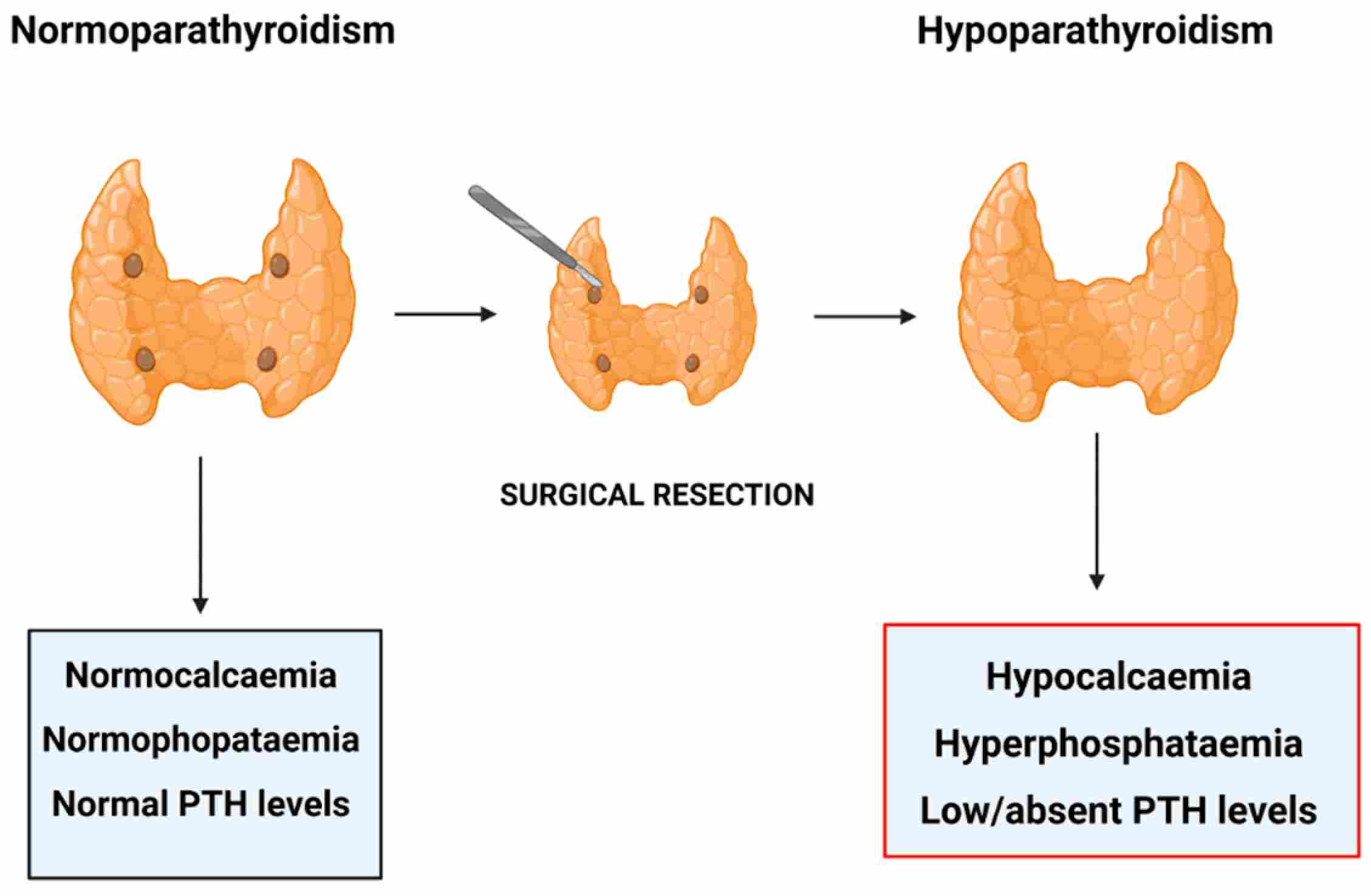 Fig.1 Post-surgical hypoparathyroidism. (Miglietta, et al., 2021)
Fig.1 Post-surgical hypoparathyroidism. (Miglietta, et al., 2021)
The diagnosis of hypoparathyroidism is initiated by confirming its defining biochemical signature—the failure of the parathyroid glands to mount an appropriate hormonal response to hypocalcemia. This critical first step, centered on two simultaneous measurements, distinguishes true gland failure from other conditions.
The Core Diagnostic Duo: Calcium and PTH
The cornerstone of diagnosis is the concurrent measurement of serum calcium and intact parathyroid hormone (PTH). While total calcium is commonly measured, ionized calcium provides a more physiologically accurate assessment, especially in patients with albumin abnormalities. The intact PTH assay is essential for measuring biologically active hormone levels at the low end of the range.
Interpreting the Pathognomonic Pattern
The diagnostic hallmark is a specific pattern: hypocalcemia with an inappropriately normal or low PTH level. This "disconnect" reveals the failure of the normal physiologic feedback loop, where low blood calcium should trigger a sharp increase in PTH secretion. In hypoparathyroidism, this compensatory response is absent or blunted.
Ruling Out "Appropriate" PTH Response
This step serves as a crucial branching point. If PTH is appropriately elevated in response to hypocalcemia, the diagnosis shifts away from primary gland failure toward conditions like vitamin D deficiency, malabsorption, or chronic kidney disease. Confirming the inappropriately low PTH pattern is therefore the essential gateway to further evaluation for hypoparathyroidism.
Following the identification of the hallmark calcium-PTH disconnect, the next critical phase involves a confirmatory panel of tests. This step serves to solidify the diagnosis by documenting the expected downstream metabolic consequences of PTH deficiency and to establish essential baselines for managing the condition and monitoring therapy. It moves from suspicion to confirmation and actionable characterization.
This aspect focuses on measuring the direct biochemical effects of absent PTH action. The key markers are serum phosphate and serum magnesium. An elevated phosphate level (hyperphosphatemia) is a classic confirmatory finding, resulting from loss of PTH-mediated renal phosphate excretion. Simultaneously, measuring magnesium is imperative, as significant hypomagnesemia can induce a reversible, functional suppression of PTH secretion, mimicking true gland failure and requiring a different therapeutic approach.
This aspect involves tests that are critical for safe long-term management rather than immediate diagnosis. Assessment of renal function (creatinine, eGFR) is essential prior to initiating calcium and vitamin D therapy, which can impact kidney health. Furthermore, obtaining a 24-hour urinary calcium excretion measurement provides a crucial baseline; in untreated hypoparathyroidism, urinary calcium is typically low, and monitoring this parameter during treatment is vital to avoid iatrogenic hypercalciuria and its associated risks of nephrolithiasis and renal damage.
A thorough clinical history is the cornerstone of etiologic differentiation in hypoparathyroidism, as it efficiently directs the diagnostic pathway. The most critical finding is a history of anterior neck surgery, which accounts for the majority of cases and often obviates further investigation. In non-surgical patients, the history guides targeted testing by revealing clues such as a family history of hypocalcemia, symptoms of autoimmune polyglandular syndromes, or developmental anomalies, ensuring a focused and rational laboratory workup. For patients without a surgical history, specific laboratory tests help identify alternative etiologies:
Alta DiagnoTech provides a comprehensive portfolio of in vitro diagnostic (IVD) solutions to support the accurate diagnosis and management of hypoparathyroidism. Our assays cover the entire diagnostic workflow—from the initial detection of the hallmark calcium-PTH disconnect, through confirmatory metabolic profiling, to specialized testing for etiological differentiation. These reliable and precise tools are designed to deliver critical insights for definitive diagnosis, severity assessment, and personalized patient management. If you have related needs, please feel free to contact us for more information or product support.
| Product Name | Technology | Application |
| Intact PTH Immunoassay Kit | Chemiluminescent Immunoassay (CLIA) | Quantitative measurement of intact PTH levels; essential for confirming the "inappropriately low" PTH signature central to diagnosis. |
| Ionized Calcium Analyzer | Ion-Selective Electrode (ISE) | Direct and accurate measurement of biologically active ionized calcium in whole blood/serum, critical for confirming true hypocalcemia. |
| Total Calcium & Phosphorus Assay Kit | Colorimetric / UV-Enzymatic Methods | Simultaneous or individual determination of total serum calcium and inorganic phosphorus for initial screening and metabolic profiling. |
| Magnesium Detection Reagent Kit | Colorimetric / Xylidyl Blue Method | Accurate measurement of serum magnesium levels to rule out hypomagnesemia as a cause of reversible PTH suppression. |
| 25-Hydroxyvitamin D CLIA Kit | Chemiluminescent Immunoassay (CLIA) | Assessment of vitamin D status to exclude deficiency as a cause of secondary hyperparathyroidism in the differential diagnosis. |
| Autoimmune Polyglandular Syndrome Antibody Panel | Immunoblot / ELISA | Detection of specific autoantibodies (e.g., interferon-ω) to aid in the diagnosis of autoimmune APS-1 as an underlying etiology. |
| Renal Function & Urinary Calcium Profile | Colorimetric / ISE / Enzymatic Methods | Comprehensive panel including serum creatinine (eGFR) and 24-hour urinary calcium measurement to assess renal health and establish treatment baselines. |
| Genetic Testing Panel for Hypocalcemia | Next-Generation Sequencing (NGS) / PCR | Targeted analysis of genes associated with hypoparathyroidism (e.g., CaSR, PTH, GATA3) for etiological diagnosis in non-surgical, familial, or syndromic cases. |
Reference
This article is for research use only. Do not use in any diagnostic or therapeutic application.
|
There is no product in your cart. |