- Home
- Resource
- Explore & Learn
- Best Practices for Utilizing Artificial Intelligence in Clinical Flow Cytometry
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Flow cytometry has become a cornerstone in the diagnosis, prognosis, and monitoring of hematologic malignancies, such as leukemia and lymphoma. This high-throughput technique enables the multiparametric analysis of single cells, providing invaluable insights into cellular behavior in a biological context. Despite its clinical importance, the analysis of flow cytometry data is time-consuming, highly dependent on skilled technicians, and prone to human error. The manual process of data interpretation, which typically involves complex pattern recognition and gating strategies, is a significant bottleneck in clinical laboratories.
Artificial intelligence (AI) offers a promising solution to these challenges, transforming how flow cytometry data is processed and interpreted. By automating labor-intensive tasks, enhancing data accuracy, and providing robust decision support, AI has the potential to significantly improve the efficiency and reliability of clinical flow cytometry. This article explores the integration of AI into clinical flow cytometry, its current applications, and the future outlook of this transformative technology.
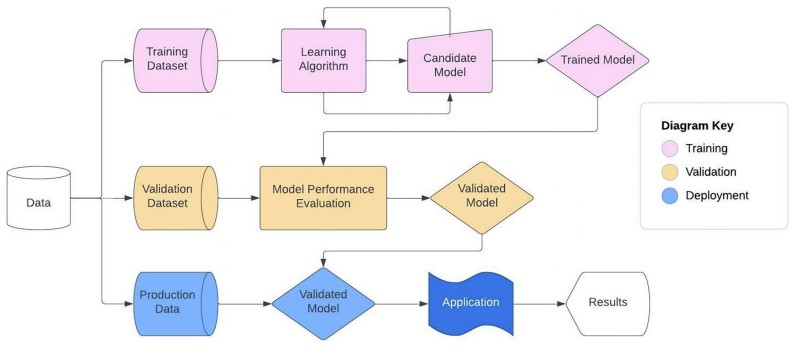 Fig.1 Machine learning model development workflow. (Ng D. P., et al., 2024)
Fig.1 Machine learning model development workflow. (Ng D. P., et al., 2024)

Flow cytometry is a powerful analytical technique that allows for the simultaneous measurement of multiple cellular parameters, such as size, granularity, and the presence of specific surface markers. It provides a detailed profile of individual cells within heterogeneous populations, making it indispensable in diagnosing various hematologic disorders. The typical flow cytometry workflow includes sample preparation, data acquisition, and analysis. However, the data analysis phase, particularly the interpretation of complex, multidimensional datasets, remains a major challenge.
The process of gating, which involves selecting specific populations of cells based on predefined criteria, requires expert knowledge and significant time investment. Moreover, the reliance on subjective judgment during data interpretation introduces variability and can lead to inconsistencies in clinical decision-making. This is especially problematic in high-stakes clinical settings, such as diagnosing acute leukemia, where rapid and accurate analysis is crucial.

Artificial intelligence, particularly machine learning (ML), holds the potential to revolutionize the way flow cytometry data is processed and interpreted. By applying advanced algorithms to flow cytometry data, AI can automate the gating process, recognize patterns in complex datasets, and provide consistent, accurate interpretations with minimal human intervention.
AI models can be trained on large, annotated datasets to recognize patterns and identify rare or previously overlooked cell populations. These models can then be applied to new data, providing real-time analysis and facilitating faster diagnosis. The ability to automate repetitive tasks not only improves workflow efficiency but also reduces the likelihood of human error, resulting in more reliable and reproducible results.
Automated Gating and Cell Population Identification
One of the most significant applications of AI in flow cytometry is the automation of gating, a task traditionally performed manually by hematopathologists. AI algorithms, such as deep learning models, can be trained to automatically identify cell populations based on a multitude of parameters, significantly reducing the time and expertise required for data analysis. These models use the inherent structure of the data to differentiate between various cell types and create more precise, reproducible gates.
AI-based gating has been shown to improve both the speed and accuracy of flow cytometry analysis. For example, deep learning techniques, including convolutional neural networks (CNNs), have demonstrated the ability to classify cell populations with a level of accuracy comparable to, or even surpassing, that of expert pathologists. This reduces the risk of missed diagnoses and enhances the overall diagnostic process.

Quality Control and Error Detection
Another promising area of AI application is in quality control (QC). Flow cytometry assays are sensitive to a variety of factors, including instrument calibration, reagent quality, and sample handling. Variations in these parameters can lead to errors in data interpretation, which may compromise diagnostic accuracy.
AI can be employed to monitor data quality in real-time, flagging any anomalies or inconsistencies that may indicate technical issues. This proactive approach to QC helps maintain the integrity of the analysis and ensures that the data being used for diagnosis is of the highest possible quality. Moreover, AI systems can identify patterns of error that may not be immediately apparent to human analysts, providing valuable insights into the underlying causes of variability.

Predictive Analytics for Prognosis and Treatment
AI is also being explored for its potential in predictive analytics, particularly in the context of hematologic malignancies. By analyzing flow cytometry data in conjunction with clinical variables, AI models can predict disease progression, treatment responses, and patient outcomes. These predictive models can assist clinicians in making more informed decisions about treatment strategies and follow-up care.
For example, AI algorithms have been used to predict minimal residual disease (MRD) levels in patients with leukemia. By analyzing multiparametric flow cytometry data, AI models can detect low-frequency tumor cells that might otherwise be missed by traditional methods, providing clinicians with a more accurate assessment of a patient’s disease status.
The adoption of AI in clinical flow cytometry requires rigorous validation to ensure that these models meet the high standards required for clinical use. Validation encompasses several key aspects, including model accuracy, reproducibility, and robustness in real-world clinical settings.
To assess the effectiveness of AI models in flow cytometry, several performance metrics must be considered. The most commonly used metrics include sensitivity, specificity, accuracy, and the area under the receiver operating characteristic curve (AUC-ROC). These metrics provide a quantitative assessment of the model's ability to correctly classify cell populations and identify abnormalities.
However, traditional performance metrics may not always be sufficient, particularly in cases involving rare diseases or imbalanced datasets. In such cases, alternative metrics such as the F1 score, precision-recall curves, and Matthews correlation coefficient (MCC) may offer a more nuanced evaluation of model performance. These metrics are particularly useful in clinical applications where minimizing false negatives (missed diagnoses) is of paramount importance, such as in the detection of rare neoplasms or acute leukemias.
Given the critical nature of flow cytometry in clinical diagnostics, a risk-based approach to validation is recommended. High-risk applications, such as automated diagnosis or treatment decision support, require more stringent validation processes compared to low-risk use cases, such as retrospective quality assurance or case flagging.
In high-risk scenarios, AI models should undergo extensive testing in real-world clinical environments, with validation performed on large, diverse datasets. Additionally, inter- and intra-laboratory variability must be assessed to ensure that AI models perform consistently across different settings and conditions.
As AI models are increasingly incorporated into clinical flow cytometry workflows, it is essential to consider the regulatory implications of these technologies. In many regions, including the United States and the European Union, software used in medical diagnostics is subject to stringent regulatory oversight.
In the context of flow cytometry, AI models that provide diagnostic or treatment recommendations may fall under the category of Software as a Medical Device (SaMD). SaMD refers to software intended for medical purposes, such as disease diagnosis or treatment planning, that does not require a physical device to function.
The regulatory framework for SaMD is still evolving, but it generally requires that AI models undergo rigorous clinical validation and performance monitoring before they can be widely adopted. The US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have issued guidelines for the approval of AI-driven diagnostic tools, emphasizing the need for robust clinical evidence and post-market surveillance to ensure patient safety.
The integration of AI into clinical flow cytometry raises several regulatory challenges, particularly in terms of data privacy, model transparency, and the potential for algorithmic bias. As AI systems are trained on large datasets, it is essential to ensure that patient data is anonymized and handled in compliance with privacy regulations such as the Health Insurance Portability and Accountability Act (HIPAA) in the United States and the General Data Protection Regulation (GDPR) in Europe.
Moreover, as AI models become more complex, ensuring transparency and explainability of their decision-making processes is critical. This will help clinicians trust AI recommendations and facilitate the identification and correction of any biases that may emerge in the models.
The future of AI in clinical flow cytometry holds immense promise, but significant challenges remain. One of the most pressing issues is the need for high-quality, annotated datasets to train AI models effectively. The availability of large, well-curated datasets will be crucial for developing AI systems that can perform accurately across diverse patient populations and disease states.
Collaboration among research institutions, healthcare providers, and regulatory bodies will be essential to overcome data-sharing challenges. Developing standardized datasets that include a broad range of disease phenotypes, patient demographics, and clinical outcomes will help ensure that AI models are robust and generalizable across different clinical settings.
Additionally, as the use of AI in flow cytometry expands, it will be necessary to develop global standards for model validation, regulatory approval, and post-market monitoring. This will ensure that AI-driven tools are safe, effective, and consistent across various healthcare systems and jurisdictions.
The adoption of AI in clinical flow cytometry will depend on the ability to explain how these models arrive at their decisions. Explainable AI (XAI) is an emerging field focused on developing models that provide transparent, understandable reasoning behind their predictions. This is particularly important in clinical settings, where healthcare professionals must have confidence in the recommendations provided by AI systems.
Efforts to improve AI explainability will not only foster trust among clinicians but also enable better integration of AI tools into clinical workflows. As AI models continue to evolve, their ability to provide clear, justifiable explanations will become increasingly important in ensuring widespread acceptance in clinical practice.
Artificial intelligence has the potential to revolutionize clinical flow cytometry by automating data analysis, improving diagnostic accuracy, and enhancing overall clinical workflows. While significant challenges remain, including the need for high-quality data, rigorous validation, and regulatory oversight, the future of AI in this field is bright. As AI models continue to evolve and become more integrated into clinical practice, they will play an increasingly important role in improving patient care and outcomes in hematologic malignancy diagnostics. The continued development of AI tools for flow cytometry, coupled with strong collaboration between stakeholders, will pave the way for a new era of precision medicine in clinical laboratories.
If you have related needs, please feel free to contact us for more information or product support.
Reference
This article is for research use only. Do not use in any diagnostic or therapeutic application.

Cat.No. GP-DQL-00203
Rotavirus Antigen Group A and Adenovirus Antigen Rapid Test Kit (Colloidal Gold)

Cat.No. GP-DQL-00206
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Card Style

Cat.No. GP-DQL-00207
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. GP-DQL-00211
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. GP-DQL-00212
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. IP-00189
Influenza A Rapid Assay Kit

Cat.No. GH-DQL-00200
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00201
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00202
Luteinizing Hormone Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00208
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. GH-DQL-00209
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit(Colloidal Gold), Strip Style

Cat.No. GH-DQL-00210
Luteinizing Hormone Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. IH-HYW-0001
hCG Pregnancy Test Strip

Cat.No. IH-HYW-0002
hCG Pregnancy Test Cassette

Cat.No. IH-HYW-0003
hCG Pregnancy Test Midstream

Cat.No. GD-QCY-0001
Cocaine (COC) Rapid Test Kit

Cat.No. GD-QCY-0002
Marijuana (THC) Rapid Test Kit

Cat.No. GD-QCY-0003
Morphine (MOP300) Rapid Test Kit

Cat.No. GD-QCY-0004
Methamphetamine (MET) Rapid Test Kit

Cat.No. GD-QCY-0005
Methylenedioxymethamphetamine ecstasy (MDMA) Rapid Test Kit

Cat.No. GD-QCY-0006
Amphetamine (AMP) Rapid Test Kit

Cat.No. GD-QCY-0007
Barbiturates (BAR) Rapid Test Kit

Cat.No. GD-QCY-0008
Benzodiazepines (BZO) Rapid Test Kit

Cat.No. GD-QCY-0009
Methadone (MTD) Rapid Test Kit

Cat.No. GD-QCY-0011
Opiate (OPI) Rapid Test Kit

Cat.No. ID-HYW-0002
Multi-Drug Test L-Cup, (5-16 Para)

Cat.No. ID-HYW-0005
Multi-Drug Rapid Test (Dipcard & Cup) with Fentanyl

Cat.No. ID-HYW-0006
Multi-Drug Rapid Test (Dipcard & Cup) without Fentanyl

Cat.No. ID-HYW-0007
Multi-Drug 2~14 Drugs Rapid Test (Dipstick & Dipcard & Cup)

Cat.No. ID-HYW-0008
Fentanyl (FYL) Rapid Test (For Prescription Use)

Cat.No. ID-HYW-0009
Fentanyl Urine Test Cassette (CLIA Waived)

Cat.No. ID-HYW-0010
Fentanyl Urine Test Cassette (Home Use)
|
There is no product in your cart. |